ANTIFUNGAL PROTECTION AND SIZING OF PAPER WITH CHITOSAN SALTS AND CELLULOSE ETHERS. PART 1, PHYSICAL EFFECTSMARIA DEL PILAR PONCE-JIM�NEZ, FERNANDO A. L�PEZ-DELLAMARY TORAL, & EZEQUIEL DELGADO FORNU�
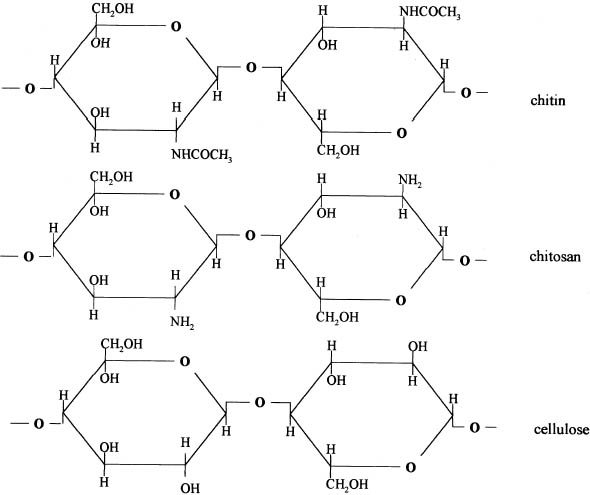
2 CHITOSANChitosan (1–4, 2-amino-2-deoxy-β-D-glucan) is a hydrophilic polyelectrolyte obtained by deacetylation of chitin. As chitin is one of the most abundant natural polymers, chitosan's potential industrial and special applications could be significant (Kienzle-Sterner et al. 1984). Chitosan is a biodegradable copolymer, consisting of 13–17% units of monomeric N-acetyl-glucosamine and 83–87% glucosamine units. It is environmentally and technologically acceptable in the repulping and recycling of paper, since structurally it is essentially cellulose with the 2-hydroxyl group replaced by a primary amino function (Allan et al. 1977) (fig. 1). The film-forming capacity of chitosan has been the object of many studies that have led to its various industrial uses—for example, in photographic films, cosmetics, and reverse osmosis membranes (Aiba et al. 1986).
The structural and stereochemical similarity between cellulose and chitosan suggests that chitosan-cellulose combinations form a chemical Tough films can be cast from these solutions. Moreover, the cationic character of the dissolved chitosan makes it substantive to the anionic fibers of paper (Domszy et al. 1985). This substantiveness can significantly improve interfiber bond, which will mediate the adverse effect of water on paper (Allan et al. 1977). Terayama (1950) obtained a water-soluble chitosan salt from crustacean shells boiled with 5 volumes of 50% sodium hydroxide (NaOH) at 60–110�C. An equal volume of filtered water was added, and the solid was dried after it was treated with monobasic acid to form a paste. The product was suitable as a water-resistant paste—for example, a paste for calico printing. Chitosan is now widely available, is relatively inexpensive, has low toxicity, and shows great potential for varied chemical derivatizations and multiple physical forms. Thus, it is an interesting compound to study in different applications. Allan et al. (1977) studied the effects of chitosan on the mechanical and optical properties of paper. They observed that in paper the best wet resistance and best dry resistance were obtained by spraying a chitosan solution on dry paper. They also did tests depositing chitosan in pulp slurries at pH 5, and then bringing them to pH 10 to precipitate the polymer on the fibers. 2.1 CHITOSAN PROPERTIESBecause of its extreme alkaline deacetylation conditions, chitosan has a shorter chain than the original chitin. It is insoluble in water, soluble in some aqueous monobasic acids, and insoluble in dilute or concentrated alkali solutions. Its solubility in aqueous mineral and organic acids is due to the formation of positively charged ammonium ions. Chitosan is insoluble in pure organic solvents, is insensitive to humidity, and contains 10–14% water. It decomposes when the temperature reaches 185�C. The solubility of chitosan in aqueous acid solutions and the viscosity of these solutions depend on the degree of acetylation and polymerization. By cautiously regulating these factors, a chitosan with 50–90% of free amino groups dissolved in a 3% acetic acid solution can vary in viscosity between 200 cps or less and 100,000 cps or more. Viscosity of chitosan solutions in an aqueous acid system can be controlled by increasing the solution temperature or by adding hydrogen peroxide or sodium peroxide. This process reduces the chain length, thereby lowering the viscosity of the solution. Conversely, the viscosity of chitosan solutions may be increased by the addition of a small quantity of formaldehyde. As greater amounts of formaldehyde are added, an increasingly insoluble gel results (Garc�a-Ochoa 1988). Apparently no experiments have been done to determine whether chitosan applied to paper is reversible. However, paper treated with chitosan is suitable for repulping and recycling (Allan et al. 1977). All additives applied as sizing agents become a structural and irreversible part of paper, including cellulose ethers. Still, cellulose ethers are acceptable for conservation works, owing to their similarity to and compatibility with cellulose. Chitosan has been shown to be even more compatible with cellulose because the differences in chemical structure between cellulose and chitosan are minimal. Because cellulose usually has a negative charge due to the presence of some carboxylate groups, it strongly interacts with the positive charges in chitosan (Allan et al. 1977). Nevertheless, more research is needed to determine the long-term effects of chitosan and any possible alterations it could cause in a variety of common inks and pigments used on paper and documents. Allan et al. (1977) performed the following tests, which give us some idea of the possible alteration of the chitosan as it ages:
These results suggest that chitosan, if sufficiently pure, will not damage the paper's appearance. |