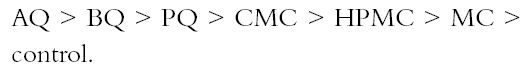ANTIFUNGAL PROTECTION AND SIZING OF PAPER WITH CHITOSAN SALTS AND CELLULOSE ETHERS. PART 1, PHYSICAL EFFECTSMARIA DEL PILAR PONCE-JIM�NEZ, FERNANDO A. L�PEZ-DELLAMARY TORAL, & EZEQUIEL DELGADO FORNU�
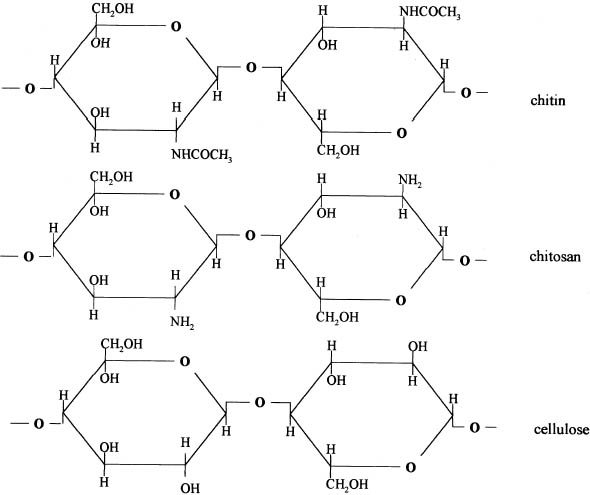
ABSTRACT—In search of alternatives for the professional conservator to use in the struggle against fungi that deteriorate paper documents, we decided to research chitosan, considering its antimolding and strengthening properties. This, the first of two articles on the topic, addresses physical results. Samples of Whatman no. 1 filter paper were coated with three chitosan salts—chitosan acetate (AQ), chitosan butyrate (BQ), and chitosan propionate (PQ)—and three cellulose ethers—sodium carboxymethylcellulose (CMC), methylcellulose (MC), and hydroxypropylmethylcellulose (HPMC). After evaluating their physical properties of folding endurance, zero-span tensile strength, brightness, and pH, both before and after sterilization with ethylene oxide (EtO), we determined that both types of products increased the zero-span strength and folding endurance of the paper. TITRE—Protection anti-fongique et encollage du papier au moyen des sels de chitosane et des �thers cellulosiques. l�re partie, les propri�t�s physiques. R�SUM�—�tant donn� ses propri�t�s antifongiques et renforcissantes, une �tude a �t� entreprise afin de d�terminer si le chitosane pourrait �tre utilis� lors du traitement des documents en papier atteints de moisissure. Ceci est le premier de deux articles sur ce sujet et il discute des effets physiques du chitosane sur le papier. Des �chantillons de papier filtre Whatman no. 1 ont �t� enduits de trois sels de chitosane, soit l'ac�tate de chitosane (AQ), le butyrate de chitosane (BQ) et le propionate de chitosane (PQ), ainsi que de trois �thers cellulosiques, soit la carboxym�thylcellulose sodique (CMC), la m�thylcellulose (MC) et l'hydroxypropylm�thyl cellulose (HPMC). Avant et apr�s st�rilisation avec de l'oxyde d'�thyl�ne, les �chantillons ont fait l'objet des tests m�caniques suivants: r�sistance au pliage, r�sistance � la traction, r�sistance � la traction lors d'essai � serrage nul. Le degr� de clart� du papier et le taux d'acidit� ont aussi �t� mesur�s. Les r�sultats d�montrent que les deux types d'enduits augmentent la r�sistance � la traction lors d'essai � serrage nul et la r�sistance au pliage du papier. TITULO—Protecci�n contra hongos y apresto de papel con sales de quitos�n y �teres de celulosa. Parte 1, efectos f�sicos. RESUMEN—En busca de alternativas de uso para el conservador profesional en la lucha contra los hongos que deterioran los documentos de papel, decidimos investigar el quitos�n, considerando que tiene propiedades fungicidas y que refuerzan el papel. En este primer art�culo, de dos que escribimos sobre el tema, se analizan los resultados f�sicos. Se recubrieron muestras de papel de filtro Whatman no. 1 con sales de quitos�n —acetato de quitos�n (AQ), butirato de quitos�n (BQ), y propionato de quitos�n (PQ)—y tres �teres de celulosa— carboximetil celulosa de sodio (CMC), metil celulosa (MC), e hidroxipropil metil celulosa (HPMC). Despu�s de evaluar las propiedades f�sicas de la resistencia al doblado, rango cero, fuerza tensil, brillo, y pH, tanto antes como despu�s de esterilizaci�n con �xido de etileno (EtO), determinamos que ambos tipos de productos aumentan la fuerza de rango cero y la fuerza de doblado del papel. 1 INTRODUCTIONThis article examines materials for sizing mold-damaged paper supports. The work is divided into two parts. The first part deals with the sizing behavior of chitosan salts and cellulose ethers on the physical and mechanical properties of Whatman no. 1 filter paper. The second part covers the antifungal protection offered by these same substances against a select group of fungal strains. The paper conservator is sometimes confronted with the problem of restoring documents that have fungal deterioration. A wide variety of fungi inhabit libraries and archives, and many are resistant to changes in humidity and temperature. The enzymes in fungi gradually destroy the cellulose, hemicellulose, lignin, and other substances that make up documents, books, and works of art on paper. The depolymerization of cellulose results in visible deterioration in the form of staining and weakened fibers. Therefore, the paper conservator who Various methods can be used to solve the problem of paper weakening. Many polymers applied as a coating can improve the strength of paper, although, over time, micro-organisms can still damage materials treated in this fashion. There are some products, however, that are both effective for sizing and resistant to fungi attack. For example, polyethylene-coated paper shows no evidence of micro-organism growth, and parylene has been shown to protect paper and improve its biostability (Dobroussina et al. 1996). A multitude of studies have revealed a variety of efficacious materials. This article will evaluate chitosan, which has not been previously analyzed for conservation use. Chitosan is a substance obtained by deacetylation of chitin, the principal component of many living forms, including fungi, insects, and crustaceans (Muzzarelli 1977). Chitosan is a whitish, amorphous powder with a crystalline structure similar to that of pure chitin. It is an interesting product because of its properties, abundance, and multiple uses, but until now there have been no investigations into its application to paper conservation. Cellulose ethers have been widely studied as consolidating agents for paper (Feller and Wilt 1990), obviating the need to describe their advantages and disadvantages in this article, though it is noteworthy that cellulose ether–treated papers can be prone to fungi attack, depending on storage conditions. In this study, we compare some properties of Whatman no. 1 filter paper samples consolidated with 2% aqueous solutions (w/v) of three cellulose ethers (sodium carboxymethylcellulose [CMC], methylcellulose [MC], and hydroxypropylmethylcellulose [HPMC]) and with three 2% aqueous solutions (w/v) of chitosan salts (acetate [AQ], butyrate [BQ], and propionate [PQ] of chitosan). 2 CHITOSANChitosan (1–4, 2-amino-2-deoxy-β-D-glucan) is a hydrophilic polyelectrolyte obtained by deacetylation of chitin. As chitin is one of the most abundant natural polymers, chitosan's potential industrial and special applications could be significant (Kienzle-Sterner et al. 1984). Chitosan is a biodegradable copolymer, consisting of 13–17% units of monomeric N-acetyl-glucosamine and 83–87% glucosamine units. It is environmentally and technologically acceptable in the repulping and recycling of paper, since structurally it is essentially cellulose with the 2-hydroxyl group replaced by a primary amino function (Allan et al. 1977) (fig. 1). The film-forming capacity of chitosan has been the object of many studies that have led to its various industrial uses—for example, in photographic films, cosmetics, and reverse osmosis membranes (Aiba et al. 1986).
The structural and stereochemical similarity between cellulose and chitosan suggests that chitosan-cellulose combinations form a chemical Tough films can be cast from these solutions. Moreover, the cationic character of the dissolved chitosan makes it substantive to the anionic fibers of paper (Domszy et al. 1985). This substantiveness can significantly improve interfiber bond, which will mediate the adverse effect of water on paper (Allan et al. 1977). Terayama (1950) obtained a water-soluble chitosan salt from crustacean shells boiled with 5 volumes of 50% sodium hydroxide (NaOH) at 60–110�C. An equal volume of filtered water was added, and the solid was dried after it was treated with monobasic acid to form a paste. The product was suitable as a water-resistant paste—for example, a paste for calico printing. Chitosan is now widely available, is relatively inexpensive, has low toxicity, and shows great potential for varied chemical derivatizations and multiple physical forms. Thus, it is an interesting compound to study in different applications. Allan et al. (1977) studied the effects of chitosan on the mechanical and optical properties of paper. They observed that in paper the best wet resistance and best dry resistance were obtained by spraying a chitosan solution on dry paper. They also did tests depositing chitosan in pulp slurries at pH 5, and then bringing them to pH 10 to precipitate the polymer on the fibers. 2.1 CHITOSAN PROPERTIESBecause of its extreme alkaline deacetylation conditions, chitosan has a shorter chain than the original chitin. It is insoluble in water, soluble in some aqueous monobasic acids, and insoluble in dilute or concentrated alkali solutions. Its solubility in aqueous mineral and organic acids is due to the formation of positively charged ammonium ions. Chitosan is insoluble in pure organic solvents, is insensitive to humidity, and contains 10–14% water. It decomposes when the temperature reaches 185�C. The solubility of chitosan in aqueous acid solutions and the viscosity of these solutions depend on the degree of acetylation and polymerization. By cautiously regulating these factors, a chitosan with 50–90% of free amino groups dissolved in a 3% acetic acid solution can vary in viscosity between 200 cps or less and 100,000 cps or more. Viscosity of chitosan solutions in an aqueous acid system can be controlled by increasing the solution temperature or by adding hydrogen peroxide or sodium peroxide. This process reduces the chain length, thereby lowering the viscosity of the solution. Conversely, the viscosity of chitosan solutions may be increased by the addition of a small quantity of formaldehyde. As greater amounts of formaldehyde are added, an increasingly insoluble gel results (Garc�a-Ochoa 1988). Apparently no experiments have been done to determine whether chitosan applied to paper is reversible. However, paper treated with chitosan is suitable for repulping and recycling (Allan et al. 1977). All additives applied as sizing agents become a structural and irreversible part of paper, including cellulose ethers. Still, cellulose ethers are acceptable for conservation works, owing to their similarity to and compatibility with cellulose. Chitosan has been shown to be even more compatible with cellulose because the differences in chemical structure between cellulose and chitosan are minimal. Because cellulose usually has a negative charge due to the presence of some carboxylate groups, it strongly interacts with the positive charges in chitosan (Allan et al. 1977). Nevertheless, more research is needed to determine the long-term effects of chitosan and any possible alterations it could cause in a variety of common inks and pigments used on paper and documents. Allan et al. (1977) performed the following tests, which give us some idea of the possible alteration of the chitosan as it ages:
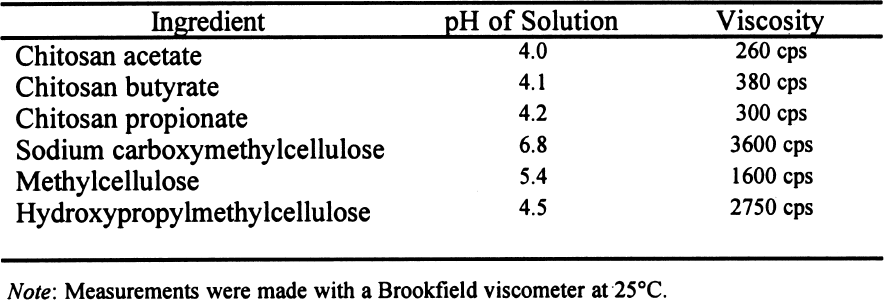
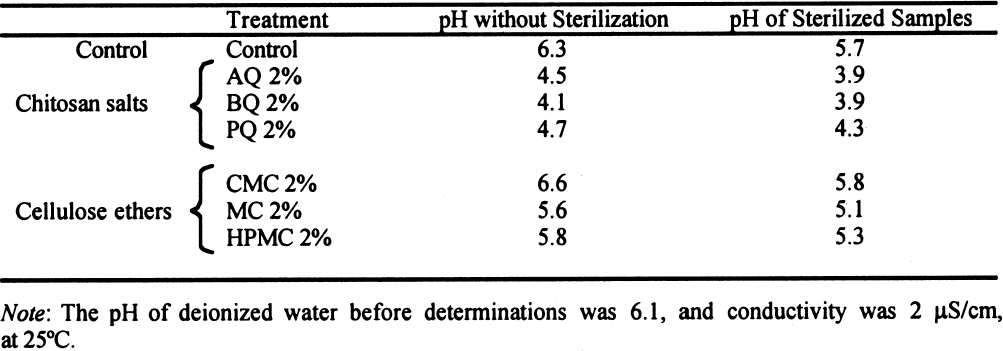
These results suggest that chitosan, if sufficiently pure, will not damage the paper's appearance. 3 MATERIALS AND METHODS3.1 SAMPLES OF PAPERWhatman no. 1 filter paper was used in these experiments because it is a pure cellulose paper that is additive-free and serves as a point of reference in respect to other experimental works. The choice of paper samples was made in accordance with TAPPI standards T400 om-90 and T402 om-93 (TAPPI 1990; TAPPI 1993). Folding endurance was tested with strips of paper 10 cm long by 1.5 cm wide, in accordance with TAPPI standard T423 om-89 (TAPPI 1989). For the brightness test, octagons of 420 mm2 were used for each sample. 3.2 SIZING OF PAPER AND STERILIZATIONThe samples were injected with 2% solutions (w/v) of each of the polymers indicated. The chitosan was prepared in the chemistry laboratory of Karl Augustin Grellman Institute of Wood, Cellulose, and Paper, University of Guadalajara, from deproteinized and decalcified shrimp shells. The shells had been treated with alkali in a two-step process to diminish the presence of N-acetyl groups (percentage of free primary amines) (Wolfrom et al. 1958). The acetylation degree of the sample was 17.3%; the viscosity (measured by a capillary viscometer) of a 1% (w/v) in 1% (v/v) acetic acid solution was 51 cps;and the sample's content in ashes was 0.23%. This chitosan was whiter, purer, and of better quality than commercial chitosan. Three different 2% (w/v) solutions of chitosan were used, employing in each case 1% glacial acetic acid, butyric acid, and propionic acid to dissolve the chitosan. Therefore, we prepared three products: chitosan acetate, chitosan butyrate, and chitosan propionate. The preparation procedure involved adding 2 g of shredded chitosan to 100 ml of deionized water. The mixture was then stirred continuously while 1 ml of the corresponding acid was slowly added to completely dissolve the chitosan. To prepare 2% solutions of the cellulose ethers CMC and HPMC, 2 g of powder were added slowly to 100 ml of deionized water, which was stirred continuously to avoid lump formation. In the case of HPMC, cold water was better for the dissolution. To obtain a homogeneous solution of the third cellulose ether, MC, 100 ml of deionized water was used: 50 ml was placed in a freezer and left to the point of freezing, and the other 50 ml was heated to the boiling point. The hot water was immediately mixed with 2 g of MC, then 50 ml of freezing water was added while the liquid was stirred vigorously. The solution was then immediately placed in a refrigerator for five minutes. A transparent viscous solution was produced. The viscosity of all 2% solutions was recorded with a Brookfield viscometer through the technique described in TAPPI standard T666 om-91 (TAPPI 1991). The pH at 25�C of all solutions was determined with a benchtop pH-meter. After the samples were coated with a roll handcoater no. 8, they were left to dry over a nylon screen. The samples were sterilized using ethylene oxide (EtO) in a sterilization chamber. This sterilization method least affected the properties of the paper. The other methods compared in the preliminary tests were sterilization in an autoclave and sterilization with ozone. The former caused significant diminution Sterilization of the samples after application of the polymer coatings was necessary to ensure that only the test organism interacted with the paper. It was necessary to evaluate the consequences of the sterilization method on the properties of the paper and coatings. 3.3 PHYSICO-MECHANICAL PROPERTIESAfter the sterilization with EtO, the modifications in paper and coating properties were evaluated in terms of folding endurance (TAPPI 1989); zerospan tensile strength (TAPPI 1985; Caulfield and Gunderson 1990); brightness (TAPPI 1992); and pH of the extracts of paper at cold temperatures (TAPPI 1988). 3.3.1 Folding EnduranceFolding endurance is directly related to fiber elasticity and to the polymer elasticity of the fiber coating. This property measures resistance to fatigue, and it has found great acceptance as a means of evaluating paper durability, that is, the degree to which the paper keeps its physical properties in relation to the frequency of use. Folding endurance measures a sheet of paper's capability of keeping its fold line without breaking in the course of repeated folding. The Schooper tester produced eight measurements for each type of coating, including the control. 3.3.2 Zero-Span Tensile StrengthTo test for zero-span tensile strength, the clips of the tester were placed as close as possible to zero separation. The aim is to test the individual fibers caught between the clips in order to show only the resistance of the fiber, not the resistance of the fiber network. (Unlike zero-span tensile strength, folding endurance is related to the intrinsic resistance of the paper and the cohesive force between fibers.) The zero-span tests were carried out with a modified pendulum-type tensile-strength machine, and strips 1.5 cm wide were utilized in accord with TAPPI T231 cm-85 (TAPPI 1985). Zero-span tensile strength reflects the intrinsic resistance of the fibers, thus the condition of the fibers. Enzymatic or age deterioration will affect the zero-span tensile strength. 3.3.3 Brightness TestBrightness tests with an Elrepho Zeiss instrument TAPPI standard T452 om-92 (TAPPI 1992) measured the reflection percentage at 457 nm and noted changes in the color of coated and uncoated paper. In this test, eight replicas of each sample were made, obtaining a mean and standard deviation. 3.3.4 Determination of pHThe final pH measure of the sample aqueous extracts was carried out using TAPPI standard T509 om-88 (TAPPI 1988), a measure that was important because acidity promotes the deterioration of the paper. These pH tests were administered to each sample before and after sterilization with ethylene oxide. 4 RESULTS4.1 VISCOSITY AND PH OF THE POLYMERIC SOLUTIONSThe viscosity of 2% solutions of chitosan is very low in comparison with cellulose ethers, thus facilitating the penetration of the solution into the paper fiber mat and making impregnation more homogeneous. All of the chitosan solutions were acidic (table 1) as a result of the preparation method of the solutions and not from any inherent characteristic of the chitosan. MC and HPMC were also acidic but could be easily adjusted by adding any alkaline agent, such as calcium hydroxide (Ca [OH]2). On the other hand, chitosan solutions will precipitate at a pH higher than 5.8.
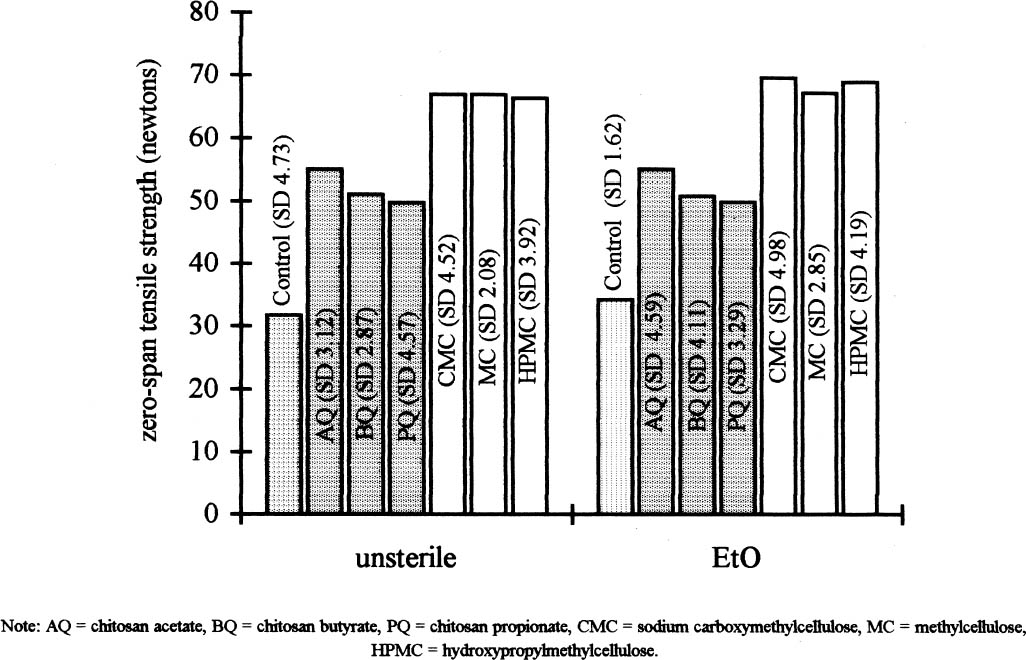
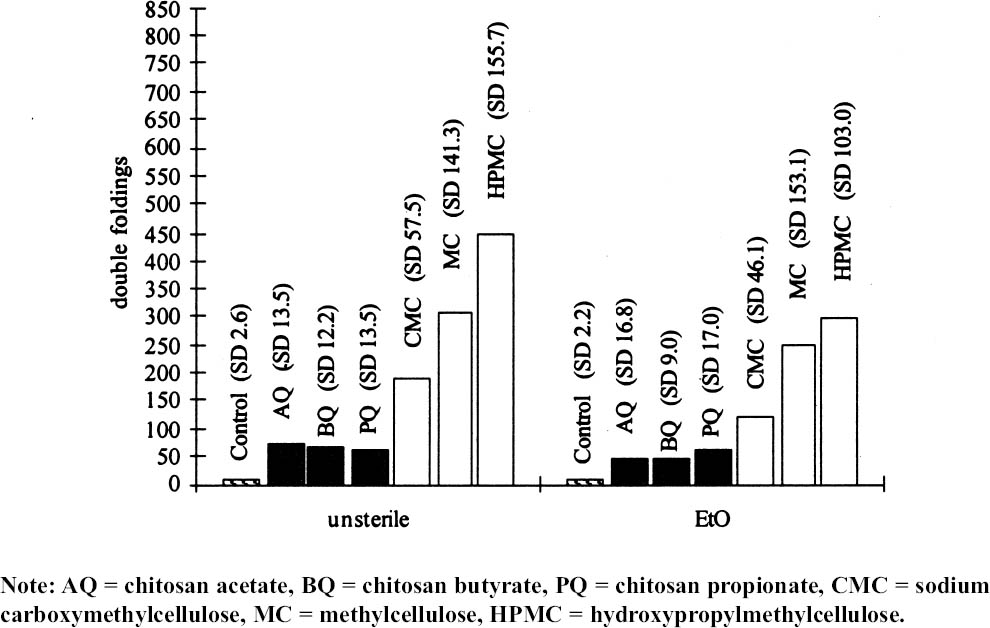
4.2 EFFECTS OF THE COATINGS ON PHYSICAL AND MECHANICAL PROPERTIES OF THE PAPERBefore the antifungal protection tests (Ponce-Jim�nez et al. 2002), the physical and mechanical properties—folding endurance, zero-span tensile strength, brightness, and pH—were evaluated to determine how the coating affected these properties of paper. Treatment with chitosan increased both the paper's zero-span tensile strength and its folding endurance. Treatment with cellulose ethers had the same effect, but to an even greater degree (figs. 2, 3). Sterilization with EtO slightly increased the zerospan tensile strength in all cases. Folding endurance increased significantly with both treatments, although cellulose ethers were more effective than chitosan. On the other hand, after sterilization with EtO, the folding endurance decreased
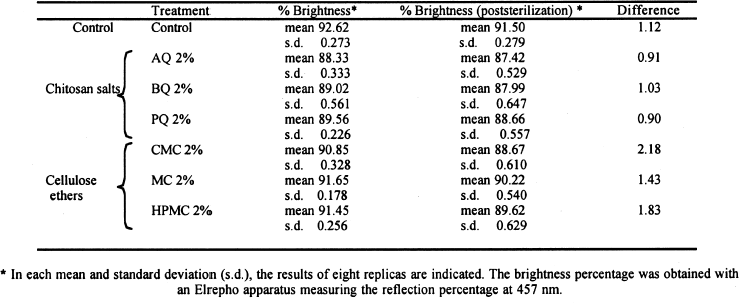
The resulting percentages of brightness before and after sterilization with EtO are shown in table 2. The last column indicates the difference between the means of the first and the second column. Chitosan salts caused more loss of paper brightness than cellulose ethers did. The sterilization with EtO slightly darkened the paper coated with either chitosan salts or cellulose ethers. The pH and viscosity using the three chitosan salts were very similar (see table 1). Cellulose ethers produced higher viscosity than the chitosan salts, and the pH was variable. Table 3 shows the results of the pH measurements of the aqueous extracts of paper before and
Chitosan salt solutions are more acidic than cellulose ether solutions. Sterilization with EtO caused a slight decrease in the pH of all the samples. 5 DISCUSSIONWhere the polymers used for coatings are concerned, the difference in viscosity between cellulose ethers and chitosan salts is evident (see table 1). This difference determines the efficacy of impregnation of the paper's fiber mat. A total impregnation can form a homogeneous and complete layer, which constitutes a good protective mechanism against micro-organisms (Guilleumas 1972). Further, the lower viscosity of chitosan salts provides better coatings than cellulose ethers. However, the results depend on the degree of polymerization, the degree of substitution (deacetylation in the case of chitosan) of the polymers involved, and the solution concentration. Another property taken into consideration was the pH of the 2% solutions. The chitosan salt solutions have a pronounced acidity because acids are employed in their formation. This acidity was inevitable because the maximum pH to solubilize chitosan was 5.7–5.8. These solutions precipitate easily at the slightest increase of pH. Neutralization probably will reinforce formation of a film on the paper's fibrous net. Allan et al. performed tests by depositioning chitosan in pulp slurries at pH 5. The slurries were then brought to pH 10 to precipitate the polymer on the fibers. But a “loss in brightness is associated with the increase in pH required to precipitate the chitosan” (Allan et al. 1977, 783). Nevertheless, it will be necessary to research the effects of a higher pH on the chitosan's ability to retard fungal growth and its long-term physical effects on the properties of the paper. To maintain paper stability, the preferred condition for preparing cellulose ethers is a neutral pH. However, the low pH of the chitosan-treated papers may be conveniently neutralized later, or a modified chitosan that is soluble in neutral aqueous solution could be used. Both of these approaches are being researched for their effects on the retardation of fungal growth and on the physical properties of the paper support, and the results will be published. Both chitosan salts and cellulose ethers increased the strength properties of Whatman no. 1 filter paper (see fig. 2). The folding endurance depends on the flexibility of the paper sheet and the film formed by the polymer applied. Both cellulose ethers and chitosan salts increase folding endurance, but chitosan to a lesser extent. This disparity is possibly due to the shorter polymer chain length of the chitosan used, as the solution's lower viscosity suggests (see table 1 and fig. 3).
Figure 3 shows that the cellulose ether coatings are superior to the chitosan in the folding test. Nevertheless, the standard deviation indicates that the coatings with cellulose ethers are more heterogeneous. Still, the chitosan has lower standard deviation even though the same method of application was Page (1969) devised a useful theory to calculate the relative contributions of the individual fiber resistance and of fiber bonding for tensile resistance. Page's theory requires measuring zero-span tensile strength, which reflects the intrinsic resistance of the paper. The standard deviations in figure 2 are lower compared with those in figure 3. The result of the test of zero-span tensile strength is the best indicator of the paper's state of conservation and is a more direct measurement of the fiber resistance. Table 2 presents the brightness averages and standard deviations before and after sterilization. Chitosan salts notably diminished the brightness of the paper, but cellulose ethers did not; the brightness value stayed within an acceptable range, with an almost invisible change. However, after sterilization with EtO, the whiteness decreased in all cases, including the control sample, and the diminishing was proportionate to the brightness value before sterilization. The only coating that maintained its brightness above 90% was MC. The chitosan used in this research was not bleached, and it likely contained impurities. Under conditions of high humidity and 28�C, a light (reddish) discoloration appeared in samples. It is unlikely that this result will impede the use of chitosan in conservation works, given that clean, high-quality chitosan with excellent transparency can be obtained (Muzzarelli et al. 1981). When the other tests were performed, it was found that the sterilization with EtO did not substantially affect the results. Zero-span tensile strength was practically unchanged, although some samples underwent overall strengthening, e.g., those coated with HPMC and CMC and the controls. There could be a cross-linkage between the polymeric chains of the cellulose and those of the cellulose ethers, a possibility that should be investigated further. In the folding endurance test, sterilization with EtO slightly reduced the elasticity of the fibers in papers coated with cellulose ethers. In the chitosancoated papers, the effect on the elasticity was less evident, particularly in the case of PQ (see fig. 3). On the other hand, the coatings' negligible effect on zero-span tensile strength reflects the resistance of the individual fibers. Table 2 shows that the sterilization diminished the brightness of the paper in one or two points. The samples with the greatest loss of whiteness after sterilization were those treated with CMC, HPMC, and MC. The second greatest loss was on the control and BQ, AQ, and PQ. This result conflicts with results before sterilization, in which the coatings diminished the brightness of the paper in the following order:
Before sterilization, the chitosan-treated papers had a greater degree of diminished brightness than the cellulose ethers had, but after sterilization with EtO, papers with cellulose ethers had a greater loss of brightness than with the chitosan salts. The results in table 3 show a decrease of pH after sterilization with EtO, which is similar in all samples except BQ and PQ. Acidity is deleterious to paper because it promotes acid hydrolysis of cellulose chains and is one of the principal mechanisms of aging and deterioration of resistance properties. This effect implies that if chitosan is used in the strengthening of paper, a deacidification of the paper would be necessary either by adding ammonia vapors in a closed chamber or finding another method that would produce a neutral pH. This need for deacidification could be decreased by making new derivatives of chitosan with neutral or slightly alkaline pH levels, which would conserve all properties with favorable characteristics. 6 CONCLUSIONSThe purpose of this project was to evaluate chitosan as a possible alternative in the consolidation of paper. In the second part of this report, we will analyze chitosan's antifungal properties (Ponce-Jim�nez et al. 2002). Based on the results obtained, we found that the 2% solutions of chitosan were more fluid and better able to impregnate paper than 2% cellulose ethers, penetrating quickly through the fibrous net. All coatings caused a lowering of brightness in paper, a result that is more evident in chitosan coatings. Conservators should use a cleaner and more transparent chitosan to achieve the best results. No degradation of zero-span tensile strength occurs as a consequence of the application of any of the polymers tested. Cellulose ethers “relax” paper and decrease the cohesion of its fiber network. On the other hand, chitosan reinforces the wet and dry strength immediately, providing an advantage for conservators when handling moist paper. No tests have been done using original documents because the long-term effects of chitosan on the paper and its effects on inks and pigments have not been investigated. It appears reasonable to use chitosan products as consolidants since they reinforce the resistance properties of paper (folding endurance and zero-span tensile strength). Using a high-quality, bleached-grade chitosan as a sizing agent is essential for achieving the minimum diminution of the optical properties of the paper. The chitosan should be applied after a thorough dry cleaning of the document surface. Also, a procedure either to neutralize the acidity or to use a neutral derivative of chitosan should be used. More research is needed, as this article represents only a first step in investigating the potential use of chitosan in the conservation field. The prospect of employing chitosan compounds as a technique of document conservation is interesting because they can be used as sizing or consolidant agents. If chitosan and its derivatives become available with a high enough purity and neutral pH, the conservator is guaranteed greater success. ACKNOWLEDGEMENTSWe wish to thank the following institutions and people for their support and for making this work possible: Karl Augustin Grellman Institute of Wood, Cellulose, and Paper, University of Guadalajara, Guadalajara, Jalisco: Jos� Turrado Saucedo, chemical engineer and head of the technology laboratory; Jos� de Jes�s Rivera Prado, chemical engineer, the bleaching laboratory; Salvador P�rez Ramos, chemical engineer, the physical-mechanical tests laboratory; Francisco Vel�zquez Cervantes, chemical pharmacology biologist, and Leticia Maya of the chemistry laboratory; Professor Bruno Becerra Aguilar of the technology area; Luz Elena Arce Castillo, chemical engineer, the library and publications area. University Center of Biological and Agronomical Sciences. Professor Rosa Mar�a Dom�nguez Arias of the University of Guadalajara. Mexican Institute of Social Security Clinic 46 in Guadalajara: Maria Luisa Preciado Quiroz and Mar�a de Jes�s Najar. Mercedes G�mez Urquiza, director of the Manuel del Castillo Negrete National School of Conservation, Restoration and Museology. Luis Daniel Mario Goeritz Rodriguez, engineer and director of the National Institute of Anthropology and History Veracruz Center. We also thank Daniel McGonagle for assistance in translating part of the text. REFERENCESAiba, S., Y.Fujiwara, T.Hideshima, C.Hwang, M.Kakizaki, M.Izume, N.Minoura, C. K.Rha, T.Shoij, A. J.Sinskey, and A.Tsutsumi. 1986. Filmogenic properties of chitin/chitosan. In Chitin in nature and technology, ed. R. A. A.Muzzarelli, C.Jeuniaux, and G. W.Gooday. New York: Plenum Press. 389–96. Allan, G. G., J. R.Fox, G. D.Crosby, and K. V.Sarkanen. 1977. Chitosan, a mediator for fiber-water interactions in paper. Seattle: College of Forest Resources, University of Washington Press.
Caulfield, D. F., and D. E.Gunderson. 1990. Paper testing and strength characteristics. In Paper preservation: Current issues and recent developments, ed. P.Luner Dobroussina, S. A. D., T. D.Velikova, and O. V.Rybalchenko. 1996. A study on the biostability of parylene-coated paper. Restaurator17:75–85. Domszy, J. G., G. K.Moore, and G. A. F.Roberts. 1985. The adsorption of chitosan on cellulose. In Cellulose and its derivatives: Chemistry, biochemistry and applications. Chichester, England, and New York: E. Horwood. 464–73. Feller, R. L., and M.Wilt. 1990. Evaluation of cellulose ethers for conservation. In Research in conservation 3. Los Angeles: Getty Conservation Institute. Garc�a-Ochoa, J. J.1988. Isolation and characterization of chitin from shrimp cuticle and transforming it into chitosan. Ph.D. diss., Guadalajara University, Guadalajara, Mexico. Guilleumas, R.1972. Disinfecting and insect elimination of bibliographical reserves in the University and Provincial Library of Barcelona. Bolet�n de la Direcci�n General de Archivos y Bibliotecas125–26:111–25. Kienzle-Sterner, C., D.Rodriguez-Sanchez, and C. K.Rha. 1984. Solution properties of chitosan chain conformation. In Chitin, chitosan and related enzymes. New York: Academic Press. 383–93. Muzzarelli, R. A. A.1977. Chitin. Oxford: Pergamon. 220–28. Muzzarelli, R. A. A., F.Tanfani, M.Emanueli, M. G.Muzzarelli, and G.Celia. 1981. The production of chitosans of superior quality. Journal of Applied Biochemistry3:316–21. Page, D. H.1969. A theory for the tensile strength of paper. TAPPI52(4):674. Ponce-Jim�nez, Maria del Pilar, Fernando A.L�pez-Dellamary Toral, and HumbertoGutierrez-Pulido. 2002. Antifungal protection and sizing of paper with chitosan salts and cellulose ethers. Part 2, Antifungal effects. Journal of the American Institute for Conservation41(2):255–68. TAPPI. 1985. Zero-span breaking length of pulp, T231 cm-85. Atlanta: Technical Association of the Pulp and Paper Industry. TAPPI. 1988. Hydrogen ion concentration (pH) of paper extracts (cold extraction method), T509 om-88. Atlanta: Technical Association of the Pulp and Paper Industry. TAPPI. 1989. Folding endurance of paper (Schooper type tester), T423 om-89. Atlanta: Technical Association of the Pulp and Paper Industry. TAPPI. 1990. Sampling and accepting a single lot of paper, paperboard, containerboard, or related product, T400 om-90. Atlanta: Technical Association of the Pulp and Paper Industry. TAPPI. 1991. Viscosity of adhesives using a low-shear rotating apparatus, T666 om-91. Atlanta: Technical Association of the Pulp and Paper Industry. TAPPI. 1992. Brightness of pulp, paper, and paperboard (directional reflectance at 457 nm), T452 om-92. Atlanta: Technical Association of the Pulp and Paper Industry. TAPPI. 1993. Standard conditioning and testing atmospheres for paper, board, pulp hand-sheets, and related products, T402 om-93. Atlanta: Technical Association of the Pulp and Paper Industry. Terayama, H.1950. Water-soluble chitosan. Japan (February 24):563.
Wolfrom, M. L., G. G.Maher, and A.Chaney. 1958. Chitosan nitrate. Journal of Organic Chemistry23:1990–91. SOURCES OF MATERIALSFilter paperWhatman no. 1 Catalogue number H-06648-17 Equipar, S.A. de C.V. Juan S�nchez Azcona No. 1447 Colonia del Valle, delegaci�n Benito Ju�rez Mexico, D.F. C.P. 03100 Cellulose ethersMethocel F4M Premium CMC and MC Droguer�a Cosmopolita S.A. de C.V. Av. Revoluci�n No. 1080 Col. Mixcoax Mexico City, Mexico ChitosanThe chitosan was prepared from shrimp shells by the Chemical Laboratory of the Karl Augustin Grellman Institute of Wood, Cellulose, and Paper, University of Guadalajara. Autopista Guadalajara-Nogales, kil�metro 15.5 Las Agujas Municipio de Zapopan, Jalisco, Mexico Apartado Postal 52-93, C.P. 45020, Zapopan, Jalisco, Mexico Roll hand-coaterRK Print-Coat Instruments Ltd. Royston Herts 5G8 OQZ, U.K. ViscometerBrookfield Engineering Laboratories 240 Cushing St. Stoughton, Mass. 02072 AUTHOR INFORMATIONMARIA DEL PILAR PONCE-JIM�NEZ received her biology degree from the National Autonomic University of Mexico and her master's degree from the University of Guadalajara with a specialty in cellulose and paper. Since 1990, she has been professor of conservation and restoration of paper and graphic documents at the National School of Conservation and Restoration and Museography at the Manuel del Castillo Negrete Institute of the National Institute of Anthropology and History (INAH) in Veracruz, Mexico. She also develops methods of teaching, application, and investigation in the field of paper conservation. Address: Centro INAH Veracruz, Benito Ju�rez No. 425 y 431, Zona Centro, C.P. 91700, Veracruz, Veracruz, Mexico. FERNANDO A. L�PEZ-DELLAMARY TORAL received his B.S. from the National Autonomic University of Mexico and his Ph.D. from the University of Wisconsin at Madison. He is a professor of chemistry and analytical chemistry at the University of Guadalajara; chief of the chemistry laboratory at the University of Guadalajara's Karl Augustin Grellman Institute of Wood, Cellulose, and Paper; and director of investigation projects on paper technology, biochemistry, and forestry engineering. Address: Departamento de Madera, Celulosa y Papel Karl Augustin Grellman, Apartado Postal 52–93, C.P. 45020, Zapopan, Jalisco, Mexico. EZEQUIEL DELGADO FORNU� received his B.S. in chemical engineering from the University of Guadalajara, his master's degree from the Karl Augustin Grellman Institute of Wood, Cellulose, and Paper at the University of Guadalajara, and his Ph.D. from University of Washington, Seattle, Washington, specializing in polymer chemistry and fiber science. He is a professor and researcher at the Karl Augustin Grellman Institute, specializing in chemical modification of fibers and polymers—natural and synthetic— and bioactive compounds such as chitosan. Address: Departamento de Madera, Celulosa y Papel Karl Augustin Grellman, Apartado Postal 52–93, C.P. 45020, Zapopan, Jalisco, Mexico.
 Section Index Section Index |