THE SWELLING OF ARTISTS' PAINTS IN ORGANIC SOLVENTS. PART 1, A SIMPLE METHOD FOR MEASURING THE IN-PLANE SWELLING OF UNSUPPORTED PAINT FILMSALAN PHENIX
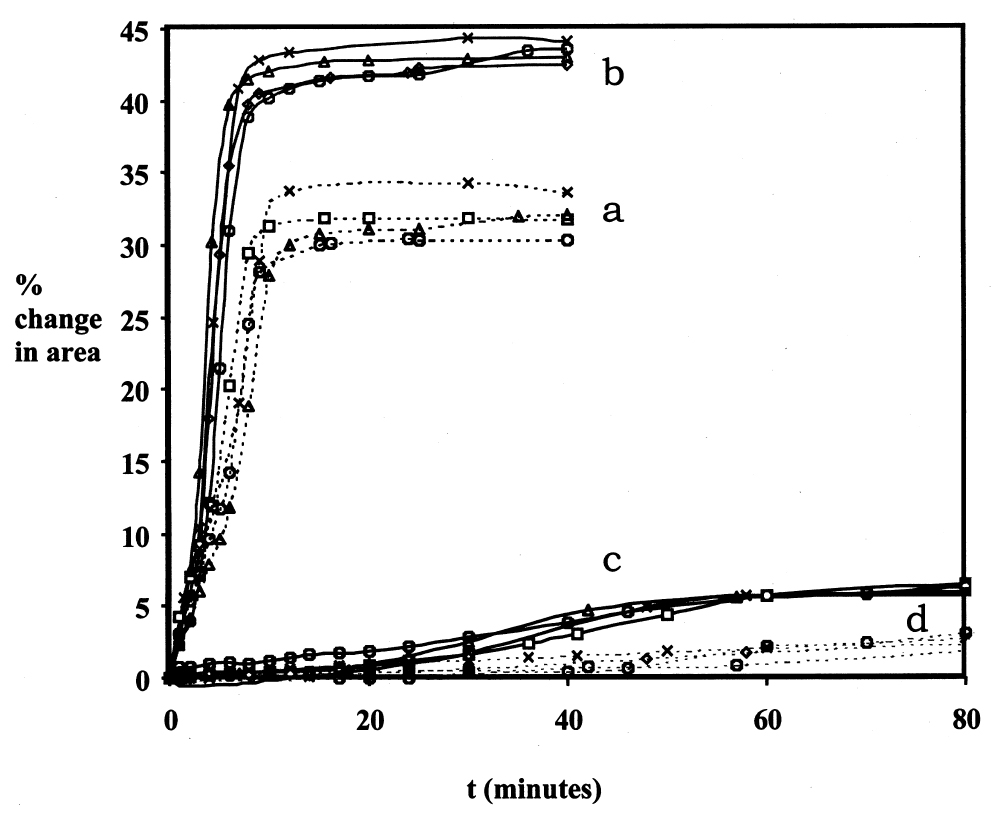
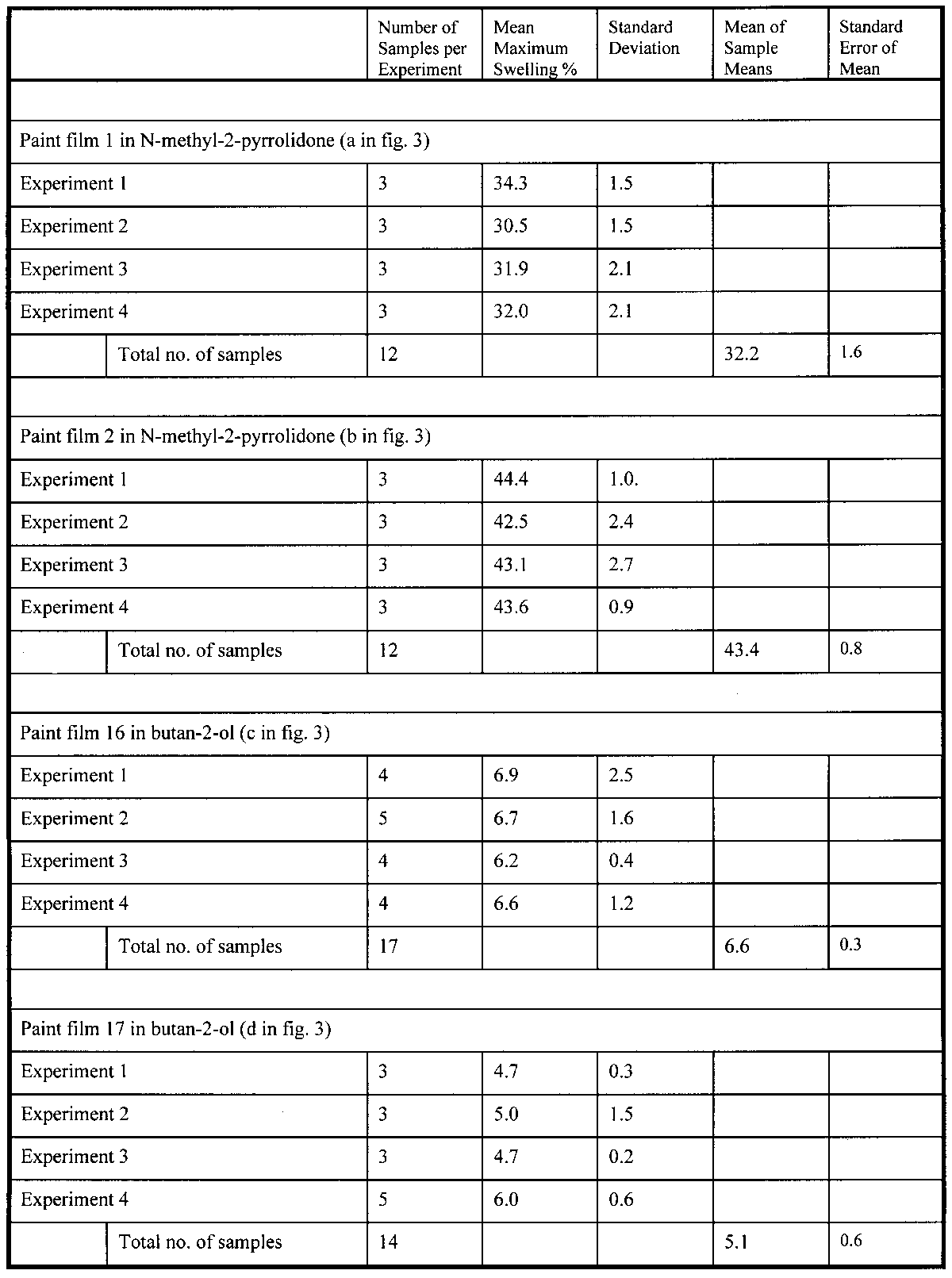
3 SOURCES OF ERRORThe technique described above measures swelling by the change in a gross physical property, particle area, due to sorption of solvent. Inevitably, a technique that is so direct and so simple in essence involves many possible sources of random and systematic error in the measurements. It is necessary here to consider the more significant issues concerning the reliability of the data, to identify the major sources of error and variability, and to discuss how these are dealt with. Three main aspects of uncertainty in the data are worthy of special consideration: temporal inconstancy of the imaging system, repeatability and accuracy, and the competing processes of leaching and swelling. 3.1 TEMPORAL INCONSTANCY OF THE IMAGING SYSTEMAn essential premise of the method is that the constant imaging conditions for all images constituting a single experiment set ensure a high degree of internal reliability in the measurement of relative paint fragment areas, both within one image frame and from one frame to the next. Put another way, within a series of images of paint particles captured over time, the dimensions of the image field of view and the boundary conditions of the particles are assumed to be constant. Any apparent change in the dimensions of a fragment image is therefore assumed to reflect a real change in actual paint fragment dimension. The absolute values of sample dimension are not, in practice, the critical parameters but rather the proportional change in dimension over time. It has been found, however, that image constancy is not perfect and that the measured area even of an inert particle can vary slightly over time. Through numerous experiments involving groups of inert particles, both immersed in liquid and unimmersed, it has been established that the inconstancy is largely systematic in character: a similar pattern and magnitude of inconstancy over time is usually observed for all particles in a group. The cause of this systematic error appears to be fluctuation in the brightness of the tungsten-halogen lamp, which leads to slight variation in boundary determination from one image to the next. The relatively low resolution of the charge coupled device (CCD) video cameras used here tends to contribute to this aspect of error due to the relatively coarse pixelation at the paint particle boundaries. It is believed that the higher resolution offered by still digital cameras will provide much greater image constancy. Future work, therefore, will use that form of image capture, rather than video, for reasons described later in the text. The temporal inconstancy is not, however, 3.2 REPEATABILITY AND ACCURACY: SAMPLE VARIABILITYVariability in measured swelling response (both rate of swelling and magnitude of maximum or equilibrium swelling) will inevitably derive from factors related to the paint fragments themselves. Size, shape, surface area, thickness, etc. will all have some influence on the specific swelling behavior of an individual paint fragment. Therefore, the source paint films for experiments of this kind must be as homogeneous as possible. An advantage of the method presented here is that several paint fragments of the same type can be tested simultaneously, giving improved statistical coverage without the need for many repeats. It is important, however, to assess the magnitude of variability within a typical sample set and the repeatability of results. The typical performance of the method in these respects is demonstrated in figure 3. The data presented in figure 3 are for two paint films (1 and 2) in the very high-swelling solvent N-methyl-2-pyrrolidone and for two paint films (16 and 17) in the low-moderate–swelling solvent butan-2-ol. For each paint-solvent combination, the mean swelling curves are shown from each of four repeat experiments. The data on maximum degree of swelling are summarized in table 2. In some instances the standard deviation of measurements of maximum % swelling within a single experiment can be relatively high in proportion with the magnitude of swelling, as in the case of the results for paint sample 16 in butan-2-ol, experiment 1. It can be seen, however, that by a relatively small number of repeat experiments the standard error can be reduced significantly1 (Miller and Miller 1993). As might be expected, the error in the measurement of maximum swelling is proportionally greater for the low-swelling cases but generally of the order of 10% of the mean value or less. This benchmark, which seems not unreasonable for a method such as this, has been used as a guide for the number of samples and repeat experiments required for particular paintsolvent combinations. 3.3 THE COMPETING PROCESSES OF LEACHING AND SWELLINGIt was demonstrated in the work of Stolow that leached paint films—that is, those from which soluble low molecular weight organic components in the paint binder have been removed by solvent extraction—usually show a different swelling response to unleached,“virgin” films (Feller et al. 1985, 61). It has been found in the present study that the phenomenon of leaching can be a significant contributor to variability in the measured swelling response of paint fragments of a given type, especially virgin films. The processes of swelling and leaching are, in some respects, in competition: increase in the dimension of a paint sample due to solvent sorption may be more or less countered by the contraction associated with
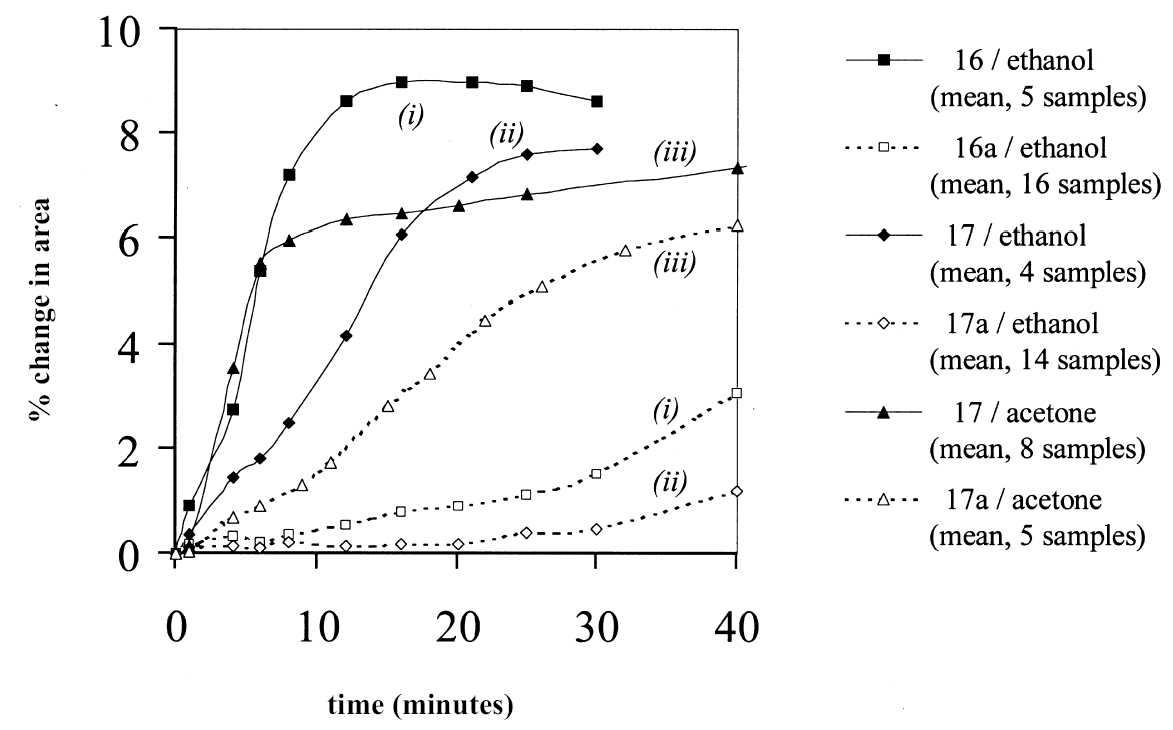
The contribution of the soluble organic phase of a paint film to the dynamic swelling response can be illustrated by comparing the swelling curves of leached and virgin paint films. Figure 4 shows the swelling behavior in ethanol of virgin samples of paint films 16 and 17 in comparison with samples from the same stock (16a and 17a) that have been extracted by immersion 10 days in acetone, followed by drying in ambient conditions for 25 days. The difference in response of the virgin and leached paint films in ethanol is really quite marked. It can be seen that, for both paint types, the preleached films swell at a much slower rate and, initially at least, to a much lesser extent than the unextracted films. Maximum degrees of swelling for the virgin paint films 16 and 17 are 9.0% and 7.7% respectively, and these values are reached quickly (in approximately 10 minutes and 25 minutes, respectively). In contrast, the preleached films have still not reached maximal/equilibrium swelling within 40 minutes, after which time the experiments are ended. The low
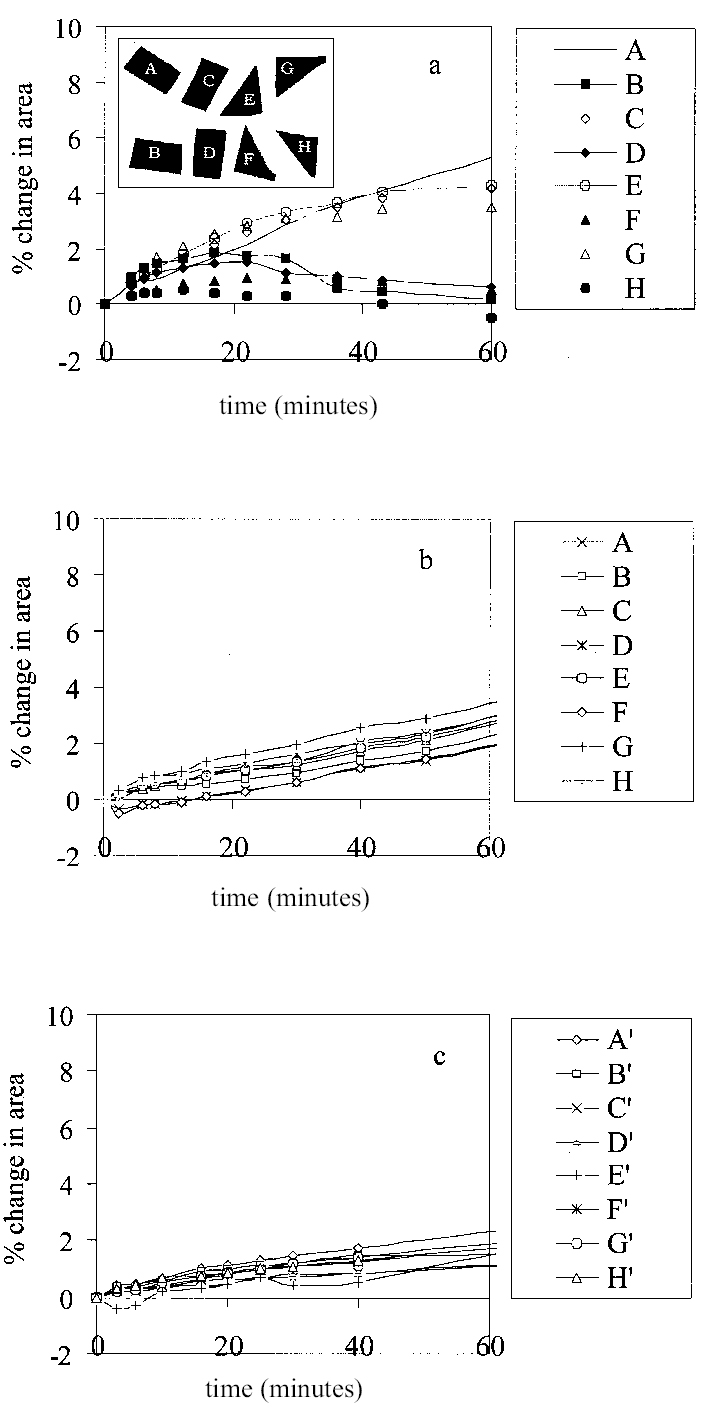
The problem leaching presents with regard to uncertainty in swelling measurements, especially on virgin paint films, is that differential rates of leaching among individual paint samples in a single experiment may contribute to appreciable variability in the measured changes in area. An experiment intended to examine this issue and the influence of sample morphology yielded results that were unexpected, but which ultimately helped to improve the experimental method. Figure 5a shows the swelling curves for eight fragments of paint film 1 (burnt umber, thermally aged) immersed simultaneously in the ether-ester solvent ethoxypropyl acetate (ethoxypropyl ethanoate). Unusually variable swelling responses had been observed in a series of experiments using this solvent, as well as other esters, and it was thought that sample shape might have been a contributing factor. As illustrated in Figure 5a, four of the paint samples were cut as rectangular
In contrast, samples B, D, F, and H initially showed a lower magnitude of swelling (< 2%), and their dynamic swelling response was characterized by a progressive decline in dimension after the maximal value had been reached, returning almost to the original area within 60 minutes—behavior that indicates leaching. This latter group of samples, which includes both triangular and rectangular shapes, all lie in the lower part of the image area, and one might look to spatial factors that could contribute to different swelling behavior of samples on one side of the “cell” compared to the other. During the course of this particular experiment, fresh solvent was delivered to the cell to compensate for solvent lost by evaporation and to dislodge bubbles that had formed around the samples. The pipette supplying the fresh solvent was located on the side of the cell closer to samples B, D, F, and H. Delivering fresh solvent to the system in this way effectively flushed the cell from one side, preferentially reducing the concentration of soluble compounds in the vicinity of samples B, D, F, and H, thereby promoting further desorption. It seems likely, therefore, that for samples B, D, F, and H leaching was accelerated and enhanced. As demonstrated earlier, the presence of soluble compounds in the paint is associated with greater degrees of swelling: therefore, preferential leaching of B, D, F, and H led to lower apparent changes in dimension. To examine if leaching had contributed to the variability in the swelling of these samples, they were left in the solvent ethoxypropyl acetate for 24 hours, after which time it was assumed they would be substantially extracted. They were then oven-dried (35�C, 24 hours), and the swelling experiment in ethoxypropyl acetate was repeated. The swelling curves for the eight samples in the repeat experiment are shown in Figure 5b. As seen previously, the swelling of the leached samples proceeds at a slower rate than does the swelling of virgin paints: the samples were still not at equilibrium after 70 minutes, when the experiment was terminated. All of the eight samples now swelled at essentially the same rate, as indicated by the gradient of the curves, and although there is some spread in the magnitude of swelling, it is comparable for all eight samples. At t = 70 minutes the mean value of % change in area is 3.13%, s.d. 0.56%, which is a more acceptable level of precision. Comparable effects were also observed when paint samples of type 1 were preextracted by immersion in acetone rather than ethoxypropyl acetate. Figure 5c shows the swelling curves, in ethoxypropyl acetate, of eight different samples (A'–H') of thermally aged burnt umber paint film 1 preleached by immersion 24 hours in acetone. Although the swelling curves are somewhat less linear than the previous case, the overall behavior is similar. The mean value of maximal swelling and the standard deviation are comparable in order of magnitude with the previous case. A key conclusion from these observations is that leaching may, with some paint-solvent combinations, be a significant contributor to experimental variability using the present method. Since addition of fresh solvent during the course of an experiment is shown to magnify the problem, this operation is avoided. In a preliminary report on this work (Phenix 1998), the low apparent swelling power of a particular, relatively polar solvent (designated solvent X) was noted. This solvent was ethoxypropyl acetate. The low values of swelling observed initially, it is now understood, were due in part to a substantial amount of leaching occurring during the swelling experiment. Ethoxypropyl acetate, as will be demonstrated in a subsequent article, is low-moderate in its swelling power on the paint films studied here (Phenix 2002, Part 2). Even so, with some of the paint films examined during this study the variability in measured values of maximum/equilibrium swelling can be so high, even with numerous repeat experiments, that the data are rather unreliable as an indicator of the magnitude of paint-solvent interaction. This conclusion applies, especially, to some of the films with very high medium content and high proportions of soluble
|