EFFECTS OF DILUTE CALCIUM WASHING TREATMENTS ON PAPERJOHN BOGAARD, & PAUL M. WHITMORE
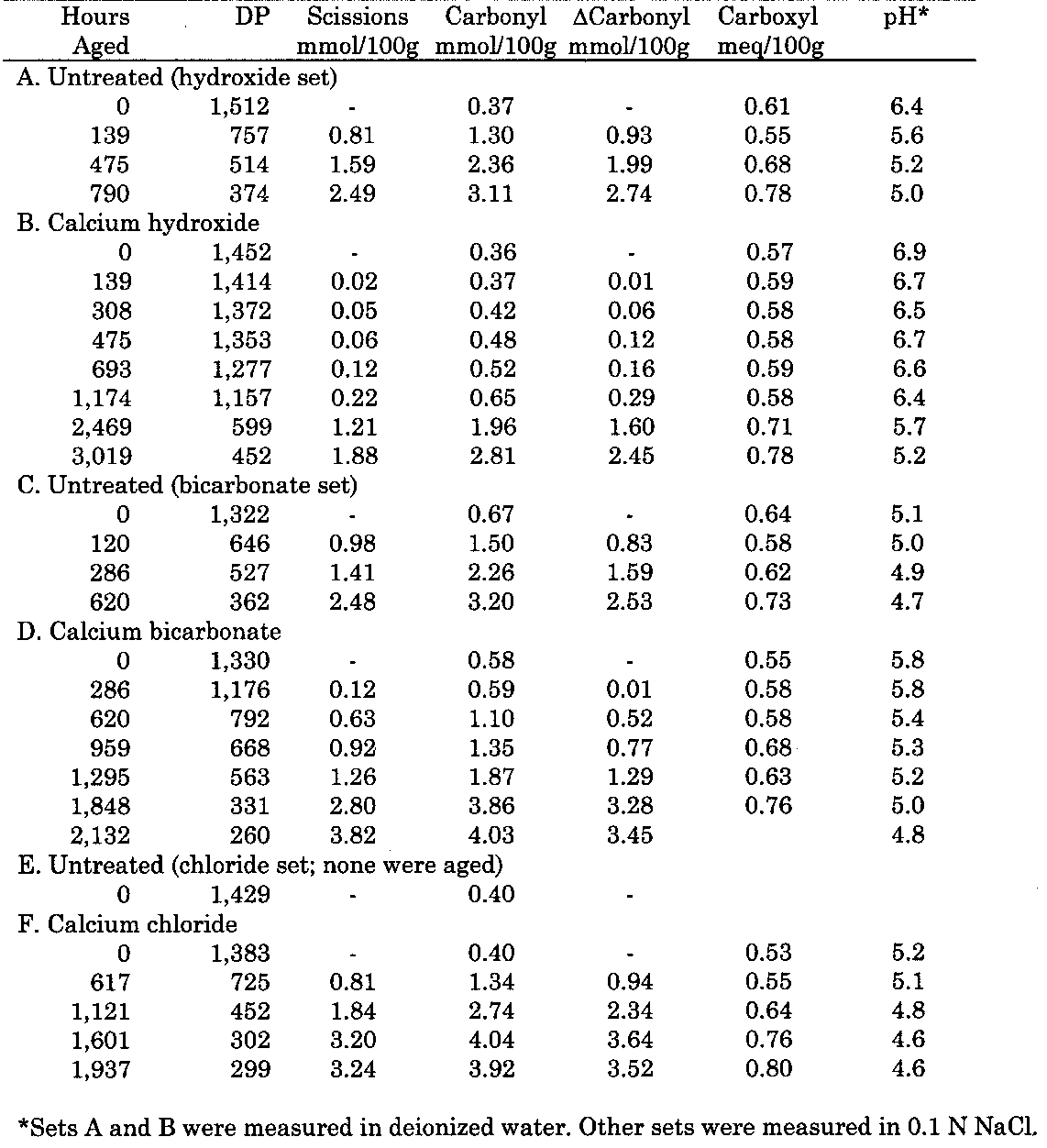
2 EXPERIMENTAL APPROACHThis study focused on the effects of three different calcium solutions on a pure cellulose paper. The three Five different sets of experiments were run on Whatman no. 42 filter paper, and they will be briefly listed here, followed by a more detailed description. In the first, a thermal aging study, naturally aged but otherwise untreated filter paper was immersed in solutions of the three calcium salts. Treated and untreated sample sheets were then aged at 90�C and 50% RH and evaluated to determine their deterioration rates. The second experiment examined the effects of alkaline degradation on unaged and photo-oxidized papers. Two sets of filter paper, one without pretreatment and the other photo-oxidized by exposure to daylight fluorescent lights, were immersed in solutions of sodium hydroxide, and their chemical products were analyzed. The third and fourth experiments investigated the effects of bath treatments on photo-oxidized paper. Filter paper sheets were photo-oxidized by exposure to ultraviolet-A radiation. In the third experiment, half of these sheets had no further treatment, while the others were treated by immersion in a dilute solution of calcium hydroxide. All the sheets were then thermally aged and evaluated. In the fourth experiment, the exposed sheets were divided into three sets: the first received no further treatment; the second was treated with sodium borohydride; the third was treated with both sodium borohydride and calcium hydroxide. All three sets were then thermally aged. In the fifth experiment, filter paper sheets were treated by immersion in calcium hydroxide or chloride. Then the treated and untreated sheets were exposed to ultraviolet-A radiation and evaluated. The treatment solutions were all prepared at the same concentration (0.625 millimolar), which was equivalent to a 40-fold dilution of a saturated calcium hydroxide solution. The literature, such as the Paper Conservation Catalog (Couch 1985) and other sources cited above, notes a range of calcium hydroxide solution strengths that have been used by conservators. The concentration of the treatment solution in this study falls somewhere in the middle of this range. The solution of calcium bicarbonate is somewhat weaker than the solution strengths used in the same literature sources. However, for comparison to other calcium salts, it was important that the same concentrations be used in all parts of the experiment. Sample sheets were treated by immersion in the calcium solution for about 10 to 15 minutes, followed by drying in the open air without restraint on polyester film (Mylar). Since the sheets were not sized, no pretreatment was necessary for their immediate thorough wetting. Calcium contents were determined by atomic absorption analysis and generally found to be around 200 ppm (by weight). Sample sets that were consistent in their calcium loading were assembled prior to accelerated aging. The details of the experimental testing are described in the appendix. Chemical tests were chosen to evaluate the samples, rather than mechanical tests, due to their higher precision and sensitivity; furthermore, cellulose degradation as measured chemically is directly related to physical property changes (Orr et al. 1954; Zou et al. 1994). The viscosity of a cellulose solution was measured in order to calculate the degree of polymerization (DP) of the cellulose chain. Colorimetric methods were used to measure both the carbonyl and carboxyl oxidation group contents. The cold extract pH was followed, and in some cases the brightness was also tracked. Treatments were carried out on either untreated filter paper or filter paper that was first photo-oxidized by exposure to ultraviolet-A lamps or high-output daylight fluorescent lights. The photo-oxidized sheets were expected to simulate the aged papers frequently encountered in conservation work. Accelerated thermal aging of treated sheets was carried out at 90�C and 50% RH, and accelerated light aging was performed under the ultraviolet-A lamps. Earlier work demonstrated that the ultraviolet-A lamps cause damage to cellulose equivalent to damage from unfiltered high-output daylight fluorescent lights, but at a much faster rate (Whitmore and
To assess the risks of these treatments, it was necessary to examine alkaline deterioration of cellulose. Alkalinity can degrade cellulose in a few different ways; however, the only reactions that proceed at a significant rate at room temperature are the elimination reactions (see, for instance, Richards 1971; Nevell 1985). One type of elimination is the peeling reaction, in which the cellulose polymer's terminal glucose unit is successively stripped away. Although peeling can continue for a large number of units before stopping, it generally has no effect on the strength of the sheet because it does not occur in the load-bearing tie chains connecting crystalline regions. The more damaging elimination reaction is the so-called β-elimination, in which links are broken To determine the chemical product distribution that characterizes alkaline degradation of photo-oxidized and unoxidized papers, samples of each were immersed in concentrated solutions of sodium hydroxide for up to 50 hours. The results of these experiments were compared with those obtained in the calcium hydroxide treatments of photo-oxidized sheets to see if the same degradation chemistries prevailed. Another approach to treating oxidized sheets is to remove the carbonyl groups, the alkali-sensitive sites on the cellulose, by chemically reducing them prior to alkaline treatment. Many articles have been published that describe different reducing agents for treating paper (see, for instance, Tang 1986; Burgess 1988; Bicchieri et al. 1999). One such compound is sodium borohydride, which is very effective at reducing the carbonyls on the cellulose chain to less reactive hydroxyls, without affecting the carboxyls. The efficacy of this approach was tested in this study by immersion of photo-oxidized papers in a solution of sodium borohydride with or without subsequent treatment of calcium hydroxide. The long-term benefits of these treatments were evaluated through accelerated thermal aging, which primarily accelerates the acid hydrolysis of cellulose (Whitmore and Bogaard 1994). Since oxidation may also play a role in the subsequent aging of treated sheets, samples in one study were exposed to ultraviolet-A radiation, which accelerates photo-oxidation. This study was limited to samples treated with calcium hydroxide or chloride, in order to explore the effects of alkaline and nonalkaline calcium treatments, compared to the light aging of untreated sheets. To more fully understand the cellulose aging chemistries, a method described in an earlier paper (Whitmore and Bogaard 1994) was employed, in which the chemical products of deterioration (chain breaks, carbonyls, and carboxyls) are analyzed. In this method, the values for the degree of polymerization are used to calculate the number of chain links that have been broken, also termed the scissions. In the present study, the scissions are tracked in concentration units (mmol/100g) that allow easy comparison with measurements of the concentrations of cellulose oxidation products. The number of scissions is compared to the measured changes in the number of carbonyl and carboxyl groups. If the numbers of scissions and carbonyls are approximately equal, with little growth in carboxyl content, then acid hydrolysis is predominant, since one aldehyde chain end is created for each hydrolytic chain break. Oxidative damage is indicated by a much greater increase in the numbers of carbonyl groups than in the number of scissions; photo-oxidation, for instance, generally produces about 2.7 carbonyl groups for every scission. Alkaline deterioration is generally indicated by an increase in carboxyl groups. A more detailed explanation of this method is contained in the appendix. The analysis is presented here in graphical plots of scissions versus carbonyls produced, since this is the most characteristic product ratio of each degradation reaction. |