PARALOID B-72 AS A STRUCTURAL ADHESIVE AND AS A BARRIER WITHIN STRUCTURAL ADHESIVE BONDS: EVALUATIONS OF STRENGTH AND REVERSIBILITYJERRY PODANY, KATHLEEN M. GARLAND, WILLIAM R. FREEMAN, & JOE ROGERS
1 INTRODUCTIONFinding appropriate adhesives for the reassembly of sculpture has challenged objects conservators for many decades. The conservator seeks a combination of properties, such as long-term stability, appropriate bond strength, ease and flexibility of application, minimal shrinkage, and, most important, maximum reversibility, that together are not the same set of characteristics typically sought by the adhesive manufacturer. In most cases it is the last characteristic—reversibility—that is lacking in modern structural adhesive formulations, since the commercial emphasis is on the lasting qualities of the bond and not on its intentional undoing. 1.1 ATTRIBUTES OF ADHESIVESThe use of more traditional animal glues and plant gum or resin-based adhesives, such as hide glue and shellac, has been superseded by the availability of modern synthetic resins, such as epoxies and polyester resins, for the reassembly of large stone sculpture. While one reason for this shift was the growing concern over the long-term stability and strength of these traditional organic-based materials, of greater influence was the ease of application offered by the newer products. One can now choose from a vast array of viscosities and working times (both pot life and cure schedule) and be assured of the reliability of a room-temperature cure and a strong bond as long as relatively simple guidelines are followed. As a result, for the last 30 to 40 years (epoxy adhesives became commercially available in the early 1950s and polyesters slightly earlier) conservators have faced trading ease of reversibility for ease of application, as well as strength and reliability. Reversibility—that is, the complete undoing of an adhesive bond with no or minimal effect on the substrates—is, however, one of the most important tenets of modern conservation. Bonds made on large-scale marble sculptures must at times be undone, either during the reassembly process or during a subsequent treatment, often many decades later. There are always risks attending any attempt to reverse a treatment, and efforts to undo bonds
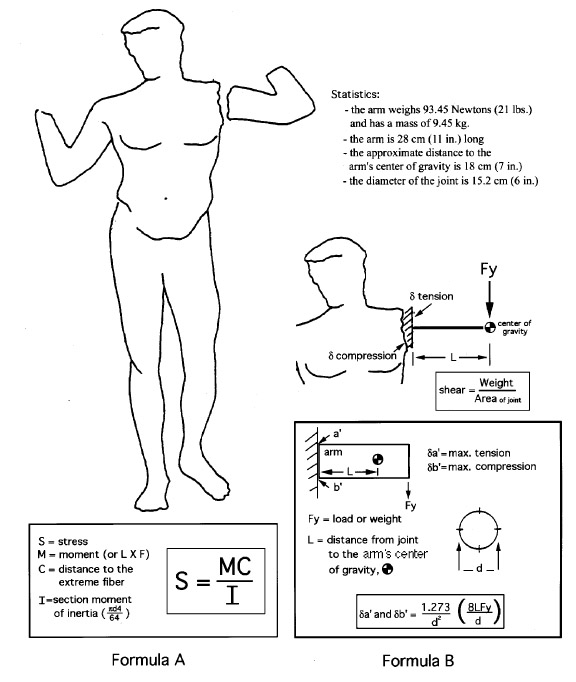
All these drawbacks have been reluctantly accepted in order to take advantage of the strength and long-term stability of these adhesives. However, a review of the characteristics of most commonly used epoxies shows that most are stronger than necessary for the applications to which the conservator puts them. For example, a bond holding an extended arm (without an internal support pin) to the shoulder of a life-size figurative marble sculpture may experience a maximum tensile stress of only 48 kPa (7 psi) and an overall shear stress on the adhesive of 5 kPa (0.75 psi) (fig. 1).This calculation is based on an arm segment with a diameter of 15.2 cm (6 in.) at the joint; a segment length of 27.9 cm (11 in.); a distance of 17.8 cm (7 in.) from the joint to the center of gravity of the segment; and a segment mass of 9.545 kg (21 lbs. or 93.45 Newtons of force). The calculation assumes that both substrates are relatively smooth and that the joint angle is close to 90 degrees. The tension, compression, and shear forces can be calculated using figure 1, formula A, a common cantilevered beam formula (Oberg et al. 1988), or a basic stress formula (fig. 1, formula B), adapted for this particular application.2 A more complex surface such as a fractured break would increase with both the surface area of the joint and
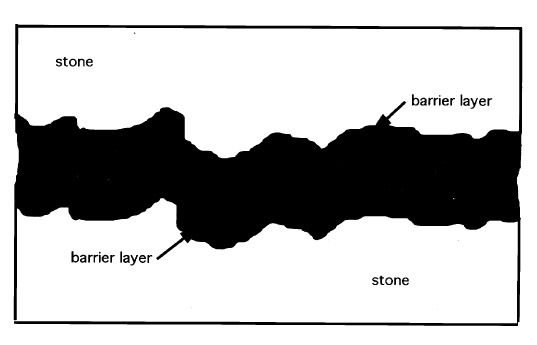
Nonetheless, while joint configurations that result in far greater stress in the bond line occur quite often, the previous example does serve to compare the required 48 kPa (7 psi) tensile strength to the common tensile strengths of most structural adhesives such as Araldite AY 103 cured with HY 991 hardener from Ciba Geigy (Ciba 1999), which provides an ultimate tensile strength of 42,749 kPa (6,200 psi). Adhesives based on unsaturated polyester resins such as Akemi Marmokitt 1000, another common adhesive used in sculpture conservation, offer tensile strengths of around 33,785 kPa (4,900 psi) or greater (Akemi 1999; Axson 1999). Clearly the strength of these two adhesive types is often greater than needed even when accommodating necessary safety factors. The conservator has little choice, however, when faced with the need for a structural bond, since nothing has yet appeared to take the place of this limited group of adhesives. Some conservators have attempted to overcome the problem of reversibility by turning to the acrylic copolymer Paraloid B-72, not as an adhesive directly, but as a barrier applied to both substrates of the joint prior to introducing a structural epoxy or polyester adhesive (Garland and Rogers 1995; Bone 1999; Bruno 1999; Christman 1999). Figure 2 illustrates a bond made with this approach. At first glance the concept has merit. The B-72 layer is readily (or more readily) soluble in acetone, which lends a degree of reversibility to the bond. The barrier layer is dissolved with minimal or no swelling of either the B-72 or the structural adhesive. However, there is an apparent flaw in this logic when one considers that the structural adhesive is simply bonding the two coatings of B-72 together with no significant penetration of one into the other (assuming there is no interaction between the structural adhesive and the acrylic copolymer to create a form of fusion bond). The bond strength is directly dependent upon the adhesion of the B-72 to the marble substrate and to the structural adhesive interlayer, as well as the cohesive strength of both. Given the strength of epoxy and polyester adhesives, the critical link, therefore, is the B-72, and in large part the integrity of the bond depends upon the strength of this material as an adhesive. Surprisingly, for a material so prevalent within the field of conservation, little information is available on the strength of B-72 as an adhesive, particularly tensile and shear evaluations. Few controlled tests following standard adhesive test procedures based specifically on the American Society of Testing and Materials' guidelines, such as ASTM D1002 for lap shear and ASTM D2095 for tensile (Pocius 1997), appear in the literature. To date the most-controlled studies have evaluated B-72 as a film (Down 1984; Down et al. 1996) rather than evaluating its “practical adhesion” characteristics as a bonding layer between two substrates. B-72 is known to soften at 30-35�C (86-95�F) and can, at that point, yield (Selwitz n.d.). Although bond failure through separation may not occur, the softened adhesive will allow movement in the bond that, for a structural adhesive used in a joint to assemble large-scale stone sculpture, must be considered failure. Additionally there has been little work done on the long-term creep and cold-flow of the copolymer at room temperature under long-term stress. 1.2 SET OF STUDIESThis account is the integration of two separate studies. Shear tests using a range of adhesive combinations were undertaken by Kathleen Garland and Joe Rogers at the Nelson-Atkins Museum of Art, Kansas City, Missouri, as part of the reassembly of a large Greek marble sculpture depicting a lion. Tensile strength evaluations were undertaken at the J. Paul Getty Museum, Los Angeles, California, by Jerry Podany as a continuation of work begun in 1994 by Martha Simpson, then interning in the Department of Antiquities Conservation at the Getty Museum. This work focused upon evaluating the ultimate strength and apparent benefits of B-72 as a barrier within a structural joint. William Freeman of Allied Signal Federal Manufacturing and Technologies in Kansas City, Missouri, undertook the design and execution of the direct testing and data coordination in both cases. While differences in approach and choice of materials exist in the two studies, such as the type of marble used and minor differences in percentages of the barrier mixtures, the conclusions, when brought together, provide useful information for the evaluation of B-72 as an adhesive. The initial purpose of both studies presented here was to evaluate the strength of a bond made with B-72 barriers on marble substrates before the introduction of either a structural epoxy or polyester adhesive. The hypothesis made was that the bonds including a B-72 barrier would be similar, if not identical, in strength to those made with a high concentration B-72 solutions alone, such as a 1:1 w/w solution in acetone (Koob 1986) or toluene, both often used in conservation practice. It was also postulated that both these adhesive bonds (those made with a B-72 barrier and those made with B-72 alone) would be significantly weaker than bonds made with commercially available epoxy and polyester structural adhesives. This assumption suggested that there was little reason to include the structural adhesive between the joint where a B-72 barrier had been applied, since the strength of the bond would be fully dependent upon the strength of the barrier material and therefore equal to a B-72 adhesive bond. Whether the B-72 as an adhesive was sufficiently strong to meet the needs of a structural joint for marble substrates was unknown. The final results of the tests confirmed some parts of these assumptions but also presented surprising information about the nature of B-72 as an adhesive. They also reflect the complexity of choices presented to the conservator when an appropriate adhesive must be selected. |