OBSERVATIONS ON CYCLODODECANE AS A TEMPORARY CONSOLIDANT FOR STONEREN�E STEIN, JOCELYN KIMMEL, MICHELE MARINCOLA, & FRIEDERIKE KLEMM
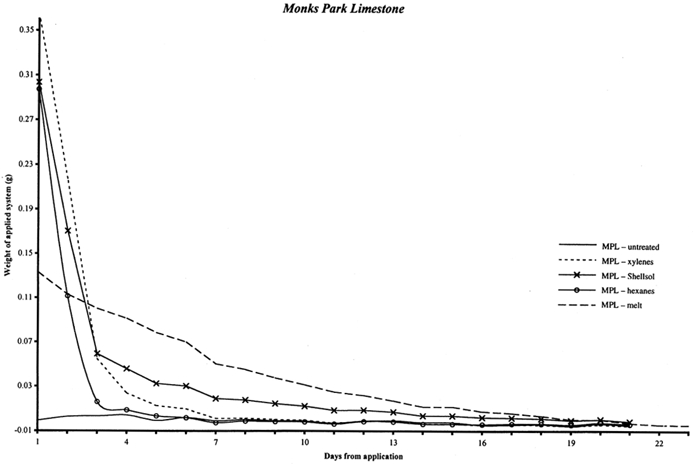
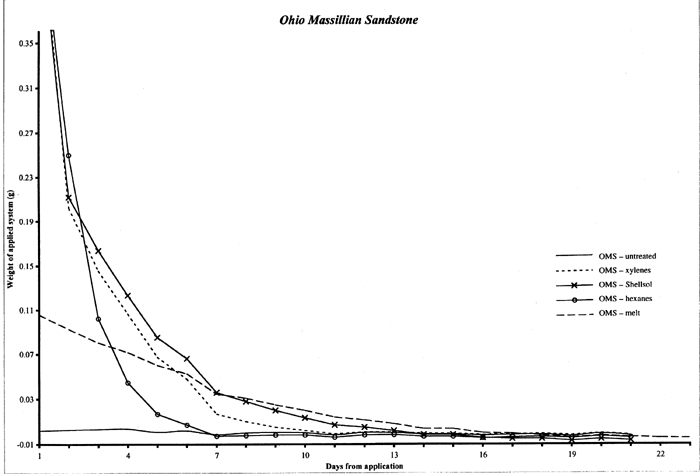
4 4. RATE OF SUBLIMATION FROM SANDSTONE AND LIMESTONESublimation of a cyclododecane coating from a nonporous glass substrate was observed to occur within two to four days, depending on the thickness and compactness of the coating. The cyclododecane coatings on the glass slides measured approximately 2 � 4 cm and ranged in thickness approximately 1 to 3 mm. In order to estimate the amount of time necessary for cyclododecane to depart from porous stone substrates, samples of Ohio Massillian sandstone and Monks Park limestone were treated with melted cyclododecane and with saturated solutions. Ohio Massillian is a sandstone building stone commonly used in the United States, while Monks Park is a fossiliferous, calcitic limestone from Bath, England. Both stone types are highly porous sedimentary stones. Mercury porosimetry was performed by Dr. George Wheeler, research chemist at the Metropolitan Museum of Art, on a single sample of each stone. Each stone exhibited a 23% open porosity, and the Monks Park limestone had a greater proportion of larger pores compared to the Ohio Massillian sandstone. Cores measuring approximately 7 cm in length and 0.5 cm in diameter were weighed to 0.00001 g on a mechanical balance. Three replicates per delivery method per stone type were prepared, and three untreated replicates of each stone type were included as controls, totaling 30 samples. Melted cyclododecane was applied with a brush to one end of the samples, and the resulting coatings measured approximately 3 mm in thickness. Approximately 0.5 ml saturated solution of cyclododecane in hexanes, Shellsol OMS, or xylenes was applied to the samples with a syringe. This volume of solution was chosen for experimental consistency, and the complete volume of 0.5 ml of consolidant solution was applied even when the samples appeared to be fully saturated. The fast evaporation rate of the more concentrated saturated hexanes solution resulted in a superficial coating of cyclododecane on the stone. The samples were weighed immediately after treatment and once each day for approximately three weeks until samples had returned to their untreated weights. The accompanying graphs record the gradual sublimation of cyclododecane, as indicated by a loss in weight to zero (fig. 3 for limestone and fig. 4 for sandstone). The weight of the untreated samples was subtracted from the treated weights, so the amount in grams of consolidant introduced by each delivery method is indicated along the y axis. Ripples in the graphed curves, particularly below zero, might be attributed to moisture uptake with fluctuations in the laboratory's relative humidity level, as is further indicated by comparable variations in the curves for the untreated samples. The initial steep declines for the solvent deliveries are interpreted as reflecting the loss of both solvent and superficial cyclododecane. The consolidant sublimes most readily from the sample surfaces, and the greatest loss of cyclododecane is therefore also likely to occur in the first days following treatment. The graphed weights then gradually diminish, presumably as cyclododecane is lost from within the stone substrate. The loss of cyclododecane from within the stone substrate is slower than the loss of consolidant from the surface. Substrate pores do allow sublimation, but they also contain the consolidant and limit its ability to sublime, similar to the effect of enclosing the coated slides in the plastic bag and box packaging. The curves illustrating those samples treated with melted cyclododecane are more linear. The melted consolidant remained on the surface of the sample, without significant penetration into the stone's pores, and is more uniformly lost with continued air exposure.
Table 1 records the number of days required for the stone samples to return to their untreated weights differentiated by application method. Sublimation periods vary in part because each delivery method introduces a different amount of cyclododecane to the stone. Because the greatest amount of cyclododecane was applied by melted delivery, the overall height of this curve is greater, and more time is needed for the consolidant to depart from the substrate. A saturated solution of cyclododecane in hexanes is approximately 140% solids, while a saturated solution in Shellsol OMS is only approximately 80%. Thus a sample treated with a given volume of hexanes solution received more cyclododecane and correspondingly required longer to return to its untreated weight.
The sublimation times also differ slightly for the two stone types, with limestone samples generally requiring more time to return to their untreated weight. Since experimental conditions were the same for both limestone and sandstone samples, this discrepancy in sublimation rate suggests that inherent stone properties, including porosity, pore size, permeability, and mineralogy, could affect both the uptake of cyclododecane and the rate of consolidant loss. In particular, the Monks Park limestone has a greater proportion of larger pores, which may promote the penetration of cyclododecane. Although, in theory, larger pore size should also allow ease of sublimation, experimental results reflect slightly longer sublimation times for the limestone samples. Further research could elucidate the relative impact of various stone properties on the penetration and subsequent sublimation of cyclododecane. Riedl and Hilbert (1998) report that after nine months of sublimation, cyclododecane was still detectable within the pores of plaster samples treated with melted cyclododecane. Prior to treatment, these plaster samples had been heated for one hour under an infrared lamp at an unspecified temperature, and up to 1 cm of penetration by the melted consolidant was achieved during application. Cyclododecane delivered via saturated solution in petroleum ether sublimed from the plaster samples within five months. Because of both the increased penetration observed on heated samples and the long sublimation times required for these samples, Riedl and Hilbert advise against heating of substrates in preparation for treatment with cyclododecane. Stone samples treated in the present study were not heated prior to treatment, and penetration of the melted consolidant was minimal, with sublimation occurring over a period of days. Discrepancies between results reported by Riedl and Hilbert and those of the present study are largely due to differences in experimental protocol. |