THE OZONE FADING OF TRADITIONAL CHINESE PLANT DYESYUN YE, LYNN G. SALMON, & GLEN R. CASS
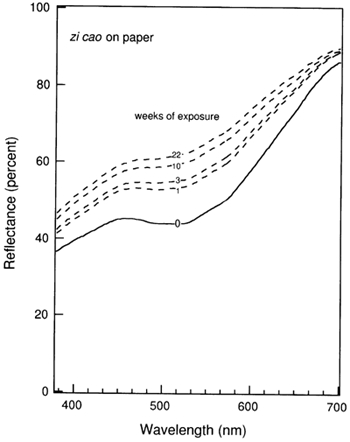
4 4. RESULTS AND DISCUSSIONThe evolution of the reflectance spectrum of the zi cao-on-paper system upon ozone exposure is illustrated in figure 1. Due to consumption of the colorant by reaction with ozone, the reflectance across the visible spectrum increases and the absorption at 510 nm is flattened. Thus, the originally pale purple system fades toward a lighter shade as the ozone exposure experiment proceeds. As seen in table 3, this sample has faded to a value of ΔE>16 by the end of the 22-week exposure, which represents a very noticeable change. The corresponding sample of zi cao on silk faded by ΔE ≌ 4 units over the course of the 22-week experiment. In general, changes in ΔE of about 2 or greater represent a change that would be perceptible by a normal human observer in side-by-side comparision (Billmeyer and Saltzman 1981).
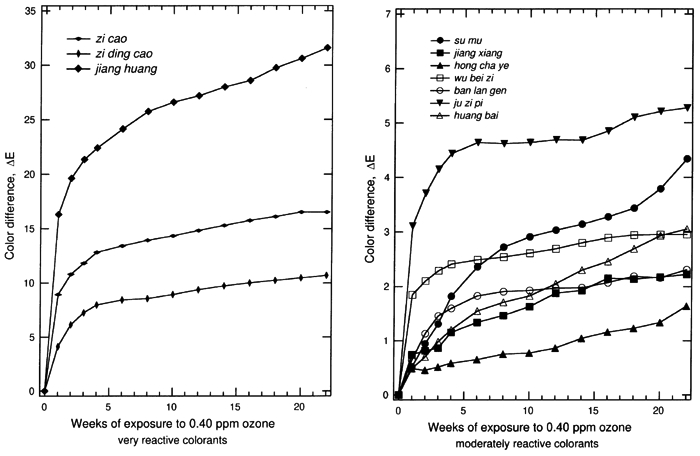
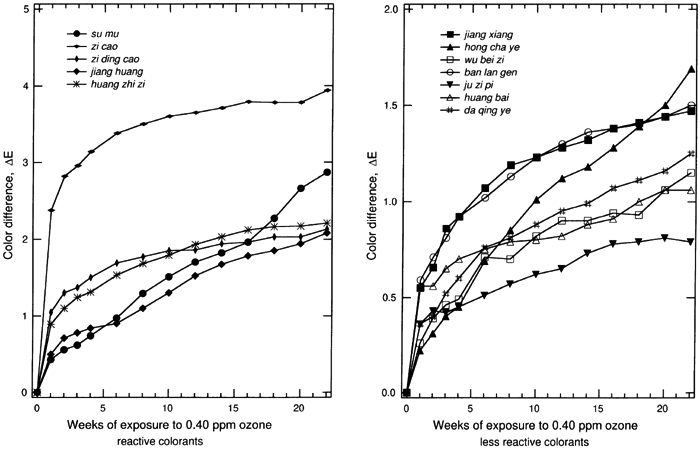
The results for the remaining samples are displayed in table 3. Most systems show a perceptible change in color (ΔE>2.0) upon exposure to 0.40 ppm ozone in the dark for 22 weeks. The total color changes, the ΔE, for colorant-on-paper systems are generally greater than the corresponding values of ΔE for the colorant-on-silk systems. This result is in part due to the fact that the colorant-on-paper systems were uniformly prepared as a surficial coating with a 30–40% reflectance at the wavelength of maximum light absorption, a scheme that is deliberately designed for the comparison of the relative ozone sensitivities in this experiment. In contrast, the dyed silk cloths were not uniformly prepared with respect to their depth of shade. (The shades were chosen by the Suzhou Silk Museum to match historical materials.) The dyed silks generally were dyed to a shade darker than the paper samples, and the dyes permeated the silk materials, making the dye less available to ozone attack. The dyes on silk also were mordanted to some extent, and that may affect the ozone-fading kinetics, as mordants have been shown to affect the light-fading mechanism of other textile-colorant systems (Gupta and Gulrajani 1996; Needles et al. 1986). The fading of these samples as a function of time during the experiment for all of the colorant-on-paper and colorant-on-silk systems is shown in figures 2 and 3, respectively. For those samples that are sensitive to ozone, much of the total color change occurs during the first week of exposure, especially for the surficial colorant deposited on paper. This result suggests that the colorant molecules on the surface of the samples, which are easily brought into contact with ozone, are consumed quickly. After the initial period of rapid reaction, the samples continuously faded during the following weeks, but with progressively smaller changes in ΔE. This slowing of the fading process over time is expected because the remaining colorant available to be reacted declines over time, while the remaining colorant embedded in the materials becomes more difficult to reach due to competition between the colorant and the substrate (either silk or paper) for destruction of the ozone as it diffuses through these materials.
Previous studies show that ozone oxidizes organic dye molecules by attacking the carbon-carbon double bonds in olefinic compounds and in some organo-metallic complexes that have aromatic structures with a partially olefinic character (Grosjean et al. 1987, 1988a, 1988b). The destruction of these double bonds, when they are part of the chromophore, causes a loss of color. The chemical mechanism of ozone reaction with alizarin lakes (Grosjean et al. 1987), with indigo (Grosjean et al. 1988a), and with curcumin (Grosjean et al. 1988b) has been studied in detail and is described elsewhere. This mechanism can be reasonably expected to explain the fading of the colorants in this study. Approximately half of the colorants examined among the set of traditional Chinese silk dyes have chemical analogs among the traditional Japanese silk dyes that have been examined previously by Paul Whitmore and Glen R. Cass (1988) to determine their ozone fading rates. Although the specific plant sources and mordants used vary between the Japanese and Chinese silk dyes, colorants based on brazilin, shikonin, crocin, berberine, curcumin, and indigo are found in both data sets. In addition, indigo blue and curcumin applied on paper have been studied previously to document their ozone fastness (Whitmore et al. 1987; Whitmore and Cass 1988). Based on comparison to these previous studies, it is possible to generalize our findings across cultures and preparation techniques. Colorant systems based on shikonin are fugitive on silk in both the Chinese and Japanese sample sets and also faded rapidly on paper in the present Chinese dyestuff experiments. Curcumin (turmeric) when applied on paper is extremely fugitive. On paper it will fade by ΔE > 30 under the conditions of our experiments, as is confirmed by exposure testing of the Chinese dye material as well as previous experiments on traditional natural organic colorants (Whitmore et al. 1987). Curiously, curcumin mordanted onto silk shows greatly reduced fading rates in both the Chinese and Japanese silk fading experiments (ΔE ≌ 2 or less). Crocin-based colorants on silk faded noticeably upon ozone exposure in the Japanese silk textile fading experiments and more slowly but still perceptibly in the Chinese silk samples. Brazilin-based colorants on silk faded to a barely perceptible degree in the Chinese silk tests and to a less than perceptible degree in the Japanese silk tests, consistent with the shorter duration of the Japanese silk fading experiments (12 weeks vs. 22 in the present study). Berberine-based colorants on silk faded to a less than perceptible extent in both the Chinese and Japanese silk fading tests. Both berberine and brazilin on paper are more fugitive than when mordanted onto silk, so these colorants should not be considered to be inert even if they fade slowly on silk. Indigo blue on paper has been shown to fade rapidly in the presence of ozone in tests of both Western watercolor pigments and Japanese art (Whitmore et al. 1987; Whitmore and Cass 1988). In contrast, the Chinese colorants ban lan gen and da qing ye, when exposed to ozone, faded to an imperceptible degree on silk, and ban lan gen faded to a barely perceptible extent on paper. Although these colorants are derived from plants that can produce indigo, neither ban lan gen nor da qing ye as tested here have the blue color that traditionally comes to mind when one thinks of indigo blue (CI no. 73000). That the colorants are not chemically the same apparently explains the slower ozone fading rate of the ban lan gen and da qing ye samples tested here when compared to indigo blue. The very rapid initial reaction of certain of these colorants holds important implications for historical dyed silk (and possibly watercolor) samples unearthed during archaeological studies. Background ozone concentrations in rural atmospheres typically average about 40 ppb (0.04 ppm), a factor of only 10 lower than the concentrations used during the present experiments. Previous ozone fading experiments involving madder lakes show that the fading rate is directly proportional to the ozone concentration multiplied by the duration of exposure (Cass et al. 1991). Thus the initial fading seen during the first week of the present experiments would be expected to occur during about the first 10 weeks if newly excavated samples were exposed directly to outdoor ambient air. To avoid such damage, the samples can be placed into storage systems that do not allow air to circulate freely over the samples, or alternatively ozone can be removed from the storage and working environment by filtration through activated carbon or by careful management of building air exchange rates (Cass et al. 1991). |