THE OZONE FADING OF TRADITIONAL CHINESE PLANT DYESYUN YE, LYNN G. SALMON, & GLEN R. CASS
ABSTRACT—ABSTRACT—Silk samples dyed with 12 traditional Chinese plant dyes were examined to measure their rate of fading upon exposure to atmospheric ozone. Samples of the same colorants extracted directly from the dried plant materials and applied to watercolor paper also were tested for their sensitivity to ozone-induced fading. The samples were exposed in a chamber to an atmosphere containing 0.40 parts per million ozone at 25�C � 1�C and 50% RH, in the absence of light, for 22 weeks. Colorant-on-paper samples produced from the dyes jiang huang (turmeric), zi cao (gromwell), and zi ding cao (violet) proved to be particularly reactive toward ozone and faded by ΔE>10 over the course of the experiment. Ju zi pi (tangerine peel extract) on paper changed color by ΔE>5, with an increase in reflectance below a wavelength of 530 nm and a decrease above 530 nm. All but one of the remaining colorant samples on paper changed by ΔE>2 as a result of ozone exposure. Color changes observed among the dyed silk samples generally were smaller than for the colorant-on-paper systems, due to their darker initial depth of shade and hence higher colorant concentration per sample, to their greater saturation of colorant throughout the cloth, and to the effects of mordanting. Colorant-on-silk samples showing color changes of ΔE>2 over the course of the experiment include zi cao (gromwell), su mu (sappan wood), huang zhi zi (gardenia), jiang huang (turmeric), and zi ding cao (violet). TITRE—L'alt�ration des colorants chinois traditionnels � base de plante lors de l'exposition � l'ozone. R�SUM�—Des �chantillons en soie teints avec douze colorants chinois traditionnels � base de plantes ont �t� examin�s pour mesurer leur degr� d'alt�ration lors de l'exposition � l'ozone atmosph�rique. Des �chantillons des m�mes colorants extraits directement des v�g�taux secs ont �t� appliqu�s sur du papier � dessin et �galement test�s pour mesurer leur sensibilit� � l'ozone. Les �chantillons ont �t� expos�s dans une �tuve � une atmosph�re contenant 0,40 parts par million d'ozone � 25oC�1oC et 50% HR, dans l'obscurit�, pendant vingt deux semaines. Les �chantillons sur papier produits � partir des colorants jiang huang (curcuma), zi cao (gr�mil) et zi ding cao (violette) se sont av�r�s �tre particuli�rement sensibles � l'ozone et ont �t� alt�r� de ΔE >10 pendant les tests. Ju zi pi(extrait de la pelure de mandarine) sur papier a chang� de couleur par ΔE >5, avec une augmentation de sa r�flectivit� au-dessous d'une longueur d'onde de 530 nm et une diminution au-dessus de 530 nm. Tous sauf un des autres �chantillons sur papier ont chang� par ΔE >2 lors des tests d'exposition � l'ozone. Les changements de couleur observ�s parmi les �chantillons en soie teints �taient g�n�ralement moins importants que ceux des �chantillons sur papier, en raison de leur teinte initiale plus fonc�e et, par cons�quent, d'une concentration plus �lev�e de colorant par �chantillon, d'une plus grande saturation du colorant dans tout le tissu et des effets du mordan�age. Les �chantillons de colorant sur soie montrant des changements de couleur de ΔE >2 au cours des tests comprennent le zi cao (gr�mil), le su mu (bois de sappan), le huang zhi zi (gard�nia), le jiang huang (curcuma) et le zi ding cao (violette). TITULO—Desvanecimiento de tintes de plantas tradicionales chinos a causa del ozono. Resumen—Muestras de seda te�idas con doce tintes de plantas tradicionales chinos fueron examinados para medir su tasa de desvanecimiento al ser expuestos al ozono atmosf�rico. Muestras de los mismos colorantes extra'dos directamente de materiales de plantas secas y aplicados a papeles para acuarelas tambi�n fueron probados para examinar sus sensibilidad al desvanecimiento inducido por el ozono. Las muestras fueron expuestas en una c�mara, a una atm�sfera que conten�a 0.40 partes por mill�n de ozono, a una temperatura de 25� C �1� C, a una humedad relativa de 50%, en ausencia de luz, y por 22 semanas. Las muestras de colorante sobre papel ΔE>10 en el curso del experimento. Ju zi pi (extracto de c�scara de naranja mandarina) sobre papel, cambi� su color en ΔE>5, y con un incremento en reflectancia bajo una longitud de onda de 530 nm y un decremento por encima de 530 nm. Todas menos una de las muestras de los colorantes sobre papel remanentes cambiaron en ΔE>2 como resultado de la exposici�n al ozono. Los cambios de color observados en las muestras de seda te�idas, generalmente fueron menores que los cambios ocurridos en el sistema de colorantes sobre papel, debido a su profundidad de sombra ΔE>2 en el curso del experimento incluyen zi cao (planta del genus Lithospermum), su mu (madera de tinte de la India), huang zhi zi (gardenia), jiang huang (c�rcuma), y zi ding cao (violeta). 1 1. INTRODUCTIONOzone is naturally present in the remote, background troposphere, where the global background ozone concentrations range from 20 to 40 parts per billion (Seinfeld and Pandis 1998). In polluted urban air, peak daily ozone levels typically range from 100 to 400 ppb (Seinfeld and Pandis 1998). Previous studies have shown that this chemically active pollutant can cause damage to organic materials. Ozone exposure can lead to cracking of natural rubber products (Newton 1945), erosion of the binders used in paints (Campbell et al. 1974), loss of tensile strength in textiles (Kerr et al. 1969), and fading of commercial anthraquinone-based synthetic textile dyes (Salvin 1969). Over the past decade, a series of experiments has shown that many of the organic colorants used by artists are particularly susceptible to ozone-induced fading. The ozone resistance of modern artists' watercolors, both organic and inorganic, has been explored (Shaver et al. 1983; Drisko et al. 1986). Further study of the traditional organic watercolor pigments used in Western art that are derived from plant and insect sources shows that many of these pigments fade rapidly in the presence of ozone at the concentrations found in today's urban atmosphere (Whitmore et al. 1987). Natural colorants that are particularly susceptible to ozone fading include madder lakes, indigos, and curcumin. The ozone-induced fading of traditional Japanese colorants applied on paper and on silk cloth and used in traditional woodblock prints has also been studied (Whitmore and Cass 1988). The traditional Chinese natural colorants remain to be examined. As is well known, China is the birthplace of sericulture. The earliest excavated silk is a group of ribbons, threads, and woven fragments, all dyed red, dated to 3000 b.c. (Kuhn 1988; Scott 1993). In recent years, thousands of silk artifacts, including silk fragments, robes, textile paintings, and tapestries, most of which are beautifully dyed, have been unearthed by archaeologists in China (Watt 1997). To protect these historic relics from oxidation and decomposition after exposure to air and light, specifications need to be established for proper storage and display. Among the environmental factors that should be considered is the risk of ozone-induced fading of the traditional Chinese colorants. The purpose of this article is to quantify the rate of ozone fading of traditional Chinese plant dyes applied on silk (and, for purposes of comparison, on paper) in order to identify ozone-sensitive colorants for which special precautions may be warranted. 2 2. TRADITIONAL CHINESE DYESPlant dyes were the dominant materials used for textile dyeing in ancient China. Chinese literary sources state that, as early as the Western Zhou Dynasty (1045–711 b.c.), a system of dyeing techniques was developed. The plant dyes used then include (Wu and Tian 1986):
During the Spring and Autumn Period (722–481 b.c.) and the Warring States Period (480–222 b.c.), mordant dying with plants containing tannic acid was widely used to yield black. The mordant employed was qing fan (iron salt) (Wu and Tian 1986). In addition to madder, safflower, which was introduced to China from the northwest tribes, became an important dyestuff during the Han Dynasty (202 b.c.–a.d. 220). It was taken to Japan in the Tang Dynasty (a.d. 618–960). During the Ming Dynasty (a.d. 1368–1644), numerous plants were employed from which dyes were extracted (Li 1981; Song 1982). In The Exploitation of the Works of Nature (Tian gong kai wu), Song Yingxing (b. 1587) reported four different plants from which indigo can be extracted, all widely planted as dyestuffs in China (Song 1982):
These natural indigos were not only used in China but also exported to Europe before synthetic organic dyes were developed (Wu and Tian 1986). More detailed descriptions of ancient Chinese dyes are not available. However, the ancient cultures of China and Japan influenced each other deeply. Sources and chemical compositions of plant dyes used in ancient Japanese textiles are presented by K�z� Hayashi (1979). Table 1 provides a brief summary of the results reported by Hayashi.
In preparation for the present experiments, samples of silk cloth dyed by traditional methods were prepared by the staff of the Suzhou Silk Museum in Suzhou, China. Staff members extracted the dyes directly from the original plant materials, and examples of both the dyed silks and the unextracted plant materials were provided for use in our experiments. The colorants furnished were identified by their Chinese names by the Suzhou Silk Museum and have been described further here based on an extensive literature review. The colorants examined are listed in table 2, and some details of their origin and properties follow.
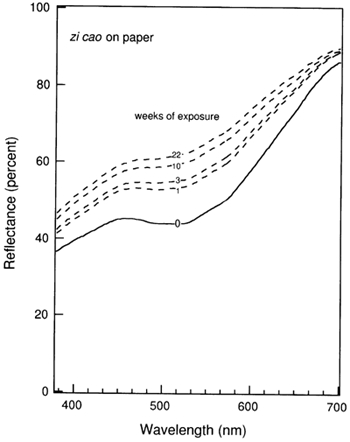
Su mu: sappan wood (Caesalpinia sappan). The sappan tree is a small evergreen tree, native to India and the Malay Peninsula, also called Brazil wood. The heartwood or the bark yields a red dye when mordanted with alum (Wee 1992). The dye constituent is brazilin (C16H14O5), the leuco-compound of brazilein (C16H12O5), which is deep red to brown in color (Pratt 1947). The mordanted dye displays good fastness toward washing (Society of Dyers and Colourists 1971). Jiang xiang: dalbergia wood (Lignum dalbergiae odoriferae). The colorant, derived from the wood of a tropical tree, yields a beige color (Wee 1992). Hong cha ye: black tea (Thea sinensis). The dye is extracted from the fermented and dried leaves of a small evergreen shrub. The main components are caffeine and tannin (10–20% gallotannic acid) (Grieve 1971). Wu bei zi: Chinese gall (Rhus chinensis). The excrescence produced by parasitic aphids on the leaves of Rhus chinensis mill, or Rhus potanimii maxim, is gathered and extracted (Liu 1988). The main constituent is gallotannin. The total tannin content is up to 70%, the richest known concentration in the plant kingdom (De Wit 1965). Zi cao: gromwell (Lithospermum erythrorhizon). The original plant source is an herb from northern China and Japan, the roots of which give a purple dye (Wee 1992). It is one of the most important mordant dyes used in ancient China. The dye constituent is shikonin, a derivative of naphthoquinone (Hayashi 1979). Ban lan gen: indigo (root) (Isatis indigotica Fort. or Isatis tinctoria L.) (Ehling and Swart 1996). The colorant is derived from the dry root of what are commonly known as indigo plants. The leaves of indigo plants contain the colorless glycoside indican (Society of Dyers and Colourists 1971), the precursor of the blue dye indigo, which has been widely studied elsewhere. The root of these plants, which is the source of the dye produced by the Suzhou Silk Museum staff, yields a yellow dye. The colorant is possibly indigo yellow (Tang 1991). Huang zhi zi: gardenia (Gardenia jasminoides Ellis). This was one of the most widely used dyes in middle China in ancient times (Needham 1954; Forbes 1987). In Records of the Historian, Sima Qian (2d century b.c.), depicts “thousands of mu [15 mu is about 1 hectare] of dyeing gardenia,” which suggests that it was a very popular dye during the Qin (221 b.c.–207 b.c.) and Han (202 b.c.–a.d. 220) Dynasties. The husks of seeds of the gardenia yield a bright yellow. The dye constituent is crocin (Hayashi 1979). Ju zi pi: tangerine peel (Citrus tangerina Hort). The Suzhou Silk Museum provided a dyed silk sample but did not provide dried plant material for ju zi pi. The yellow colorant applied by us to create the paper sample was extracted from the peel of tangerines purchased from a Chinese supermarket. Huang bai: Chinese yellow cork tree (Phellodendron amurense Rupr. or Phellodenron chinense Schneld). The deeply fissured corky bark of this deciduous tree can be extracted to yield an intense yellow. The dye constituent is berberine (Hayashi 1979). Zi ding cao: violet (Viola philippica). The extract from this herbaceous plant (Liu 1988) yields a yellowish brown color when applied on silk. Jiang huang: turmeric (Curcuma longa L.). This is a perennial plant with a stout underground stem (Wee 1992). The dye constituent is curcumin (C21H20O6), a bright yellow coloring substance that dissolves readily in water (Pratt 1947). It was an important dye in Asia as well as in Europe prior to the discovery of aniline dyes. Da qing ye: indigo (leaf) (Isatis tinctoria L.). This dye is similar to ban lan gen but is made from the unprocessed leaf of the indigo plant rather than the ground root. 3 3. EXPERIMENTSilk cloth samples, dyed with su mu, jiang xiang, hong cha ye, wu bei zi, zi cao, ban lan gen, huang zhi zi, ju zi pi, huang bai, zi ding cao, jiang huang, and da qing ye, were kindly provided by the Suzhou Silk Museum. Technical identifications of the plant materials used to make the dyes and the main components of the plant dyes are given in table 2. At the Suzhou Silk Museum, the dyes were reported to have been mordanted only with sodium chloride and acetic acid. Chemical analysis of dissolved dyed silk samples by inductively coupled plasma mass spectrometry in our laboratory showed the presence of trace amounts of calcium and iron, which may have entered into the mordanting process unintentionally. A variety of mordants are found in ancient Chinese silk, including iron, alum, copper, etc. (Zhao 1985). Information as to whether the silk was degummed was not provided. The dried plant materials from which the dyes for the paper samples were made were also obtained from the Suzhou Silk Museum unless otherwise noted. For each silk cloth sample, a small piece measuring approximately 2 x 5 cm, was cut off and sewn onto a patch of watercolor paper for easy handling. For purposes of comparison, samples of the unmordanted dyes on watercolor paper were prepared in our laboratory. Since the plant materials received were already dried, it was not easy to extract the colorants by traditional techniques. Soxhlet extraction was employed with methanol as the volatile solvent. After extraction, the solution was concentrated using a rotary evaporator before being air-brushed (Iwata HP-A) onto a 2.5 x 5 cm piece of watercolor paper (cut from Arches 140 lb. hot-pressed paper) by a technique introduced in previous work in this laboratory (Whitmore et al. 1987). An effort was made to produce samples on paper that had a reflectance of approximately 30–40% at the wavelength of maximum absorption. It has been found that with initial depths of shade in this range, the color change is sensitive to changes in the colorant concentration (Johnston-Feller et al. 1984). The colorant-on-silk samples were used as received from the Suzhou Silk Museum; they were generally dyed to a deeper shade than the colorant-on-paper samples. In contrast to the paper samples, where the colorant was applied to the surface of the paper, the silk samples were saturated with colorant throughout the depth of the cloth. The ozone exposure apparatus used in these experiments is described in detail elsewhere (Whitmore et al. 1987). Briefly, air at a flow rate of 2 lpm was purified by drawing it through a bed of activated carbon, a bed of Purafil, and a high-efficiency particle filter, followed by humidification to 50% RH. Next, the air flow was drawn through three commercial ozone generators (Ultra Violet Products SOG-2) operated in parallel. The ozonated air then was admitted to a light-tight stainless steel and glass exposure chamber. Both silk and paper samples were mounted on anodized aluminum panels, which were hung in the exposure chamber adjacent to the chamber walls, with the samples facing the interior of the box. A uniform ozone concentration was maintained within the exposure chamber by use of a magnetically coupled stirring system. The ozone concentration inside the chamber was measured continuously with a UV photometric ozone monitor (Dasibi Model 1003-PC), which was attached to a strip chart recorder. The samples were exposed to 0.40 � 0.005 parts per million ozone at 25�C � 1�C, at 50% RH, in the absence of light, for 22 weeks. Unexposed control samples of both the colorants on silk and the colorants on paper were maintained for purposes of comparison to the ozone-exposed samples. The fading rate of the colorant-on-silk and colorant-on-paper systems can be characterized by the change over time of their visible reflectance spectra. The diffuse reflectance spectra of these samples were measured with a Diano Match Scan II reflectance spectrophotometer, before and at intervals during the ozone exposure experiment. Two frequently used color notations, the CIE tristimulus values (X, Y, Z), and the CIE chromaticity coordinates and luminous reflectance (x, y, Y), calculated for CIE Illuminant C, are employed to provide information about the visual appearance of the material under the specified standard illuminant. The total perceived color difference between the samples before and after exposure to ozone can be conveniently represented by a single quantity, ΔE, which is calculated for CIE Illuminant C using the CIE 1976 L∗a∗b∗ formula (Billmeyer and Saltzman 1981). 4 4. RESULTS AND DISCUSSIONThe evolution of the reflectance spectrum of the zi cao-on-paper system upon ozone exposure is illustrated in figure 1. Due to consumption of the colorant by reaction with ozone, the reflectance across the visible spectrum increases and the absorption at 510 nm is flattened. Thus, the originally pale purple system fades toward a lighter shade as the ozone exposure experiment proceeds. As seen in table 3, this sample has faded to a value of ΔE>16 by the end of the 22-week exposure, which represents a very noticeable change. The corresponding sample of zi cao on silk faded by ΔE ≌ 4 units over the course of the 22-week experiment. In general, changes in ΔE of about 2 or greater represent a change that would be perceptible by a normal human observer in side-by-side comparision (Billmeyer and Saltzman 1981).
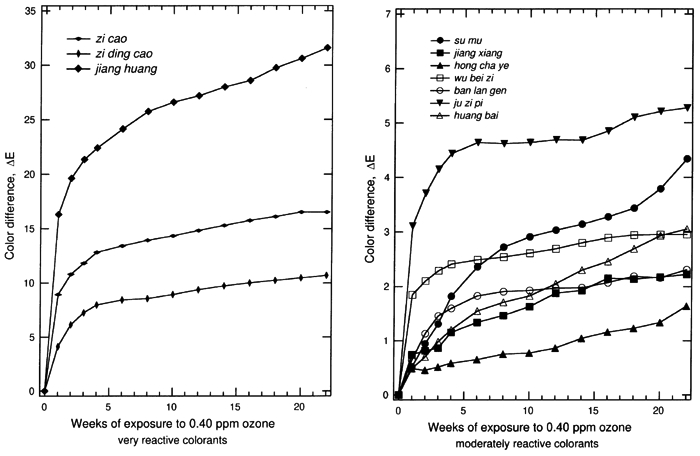
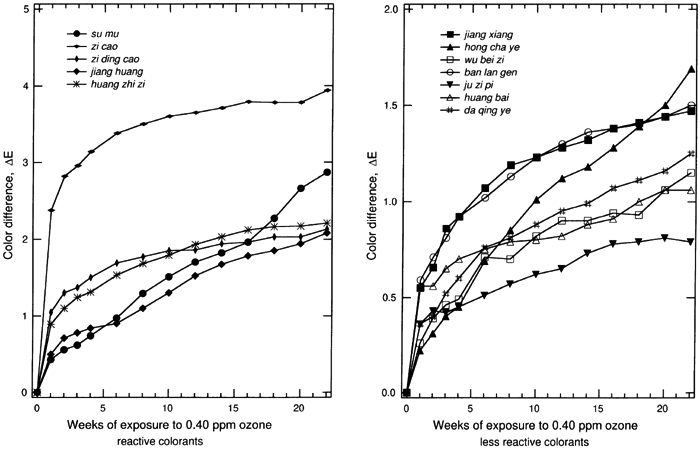
The results for the remaining samples are displayed in table 3. Most systems show a perceptible change in color (ΔE>2.0) upon exposure to 0.40 ppm ozone in the dark for 22 weeks. The total color changes, the ΔE, for colorant-on-paper systems are generally greater than the corresponding values of ΔE for the colorant-on-silk systems. This result is in part due to the fact that the colorant-on-paper systems were uniformly prepared as a surficial coating with a 30–40% reflectance at the wavelength of maximum light absorption, a scheme that is deliberately designed for the comparison of the relative ozone sensitivities in this experiment. In contrast, the dyed silk cloths were not uniformly prepared with respect to their depth of shade. (The shades were chosen by the Suzhou Silk Museum to match historical materials.) The dyed silks generally were dyed to a shade darker than the paper samples, and the dyes permeated the silk materials, making the dye less available to ozone attack. The dyes on silk also were mordanted to some extent, and that may affect the ozone-fading kinetics, as mordants have been shown to affect the light-fading mechanism of other textile-colorant systems (Gupta and Gulrajani 1996; Needles et al. 1986). The fading of these samples as a function of time during the experiment for all of the colorant-on-paper and colorant-on-silk systems is shown in figures 2 and 3, respectively. For those samples that are sensitive to ozone, much of the total color change occurs during the first week of exposure, especially for the surficial colorant deposited on paper. This result suggests that the colorant molecules on the surface of the samples, which are easily brought into contact with ozone, are consumed quickly. After the initial period of rapid reaction, the samples continuously faded during the following weeks, but with progressively smaller changes in ΔE. This slowing of the fading process over time is expected because the remaining colorant available to be reacted declines over time, while the remaining colorant embedded in the materials becomes more difficult to reach due to competition between the colorant and the substrate (either silk or paper) for destruction of the ozone as it diffuses through these materials.
Previous studies show that ozone oxidizes organic dye molecules by attacking the carbon-carbon double bonds in olefinic compounds and in some organo-metallic complexes that have aromatic structures with a partially olefinic character (Grosjean et al. 1987, 1988a, 1988b). The destruction of these double bonds, when they are part of the chromophore, causes a loss of color. The chemical mechanism of ozone reaction with alizarin lakes (Grosjean et al. 1987), with indigo (Grosjean et al. 1988a), and with curcumin (Grosjean et al. 1988b) has been studied in detail and is described elsewhere. This mechanism can be reasonably expected to explain the fading of the colorants in this study. Approximately half of the colorants examined among the set of traditional Chinese silk dyes have chemical analogs among the traditional Japanese silk dyes that have been examined previously by Paul Whitmore and Glen R. Cass (1988) to determine their ozone fading rates. Although the specific plant sources and mordants used vary between the Japanese and Chinese silk dyes, colorants based on brazilin, shikonin, crocin, berberine, curcumin, and indigo are found in both data sets. In addition, indigo blue and curcumin applied on paper have been studied previously to document their ozone fastness (Whitmore et al. 1987; Whitmore and Cass 1988). Based on comparison to these previous studies, it is possible to generalize our findings across cultures and preparation techniques. Colorant systems based on shikonin are fugitive on silk in both the Chinese and Japanese sample sets and also faded rapidly on paper in the present Chinese dyestuff experiments. Curcumin (turmeric) when applied on paper is extremely fugitive. On paper it will fade by ΔE > 30 under the conditions of our experiments, as is confirmed by exposure testing of the Chinese dye material as well as previous experiments on traditional natural organic colorants (Whitmore et al. 1987). Curiously, curcumin mordanted onto silk shows greatly reduced fading rates in both the Chinese and Japanese silk fading experiments (ΔE ≌ 2 or less). Crocin-based colorants on silk faded noticeably upon ozone exposure in the Japanese silk textile fading experiments and more slowly but still perceptibly in the Chinese silk samples. Brazilin-based colorants on silk faded to a barely perceptible degree in the Chinese silk tests and to a less than perceptible degree in the Japanese silk tests, consistent with the shorter duration of the Japanese silk fading experiments (12 weeks vs. 22 in the present study). Berberine-based colorants on silk faded to a less than perceptible extent in both the Chinese and Japanese silk fading tests. Both berberine and brazilin on paper are more fugitive than when mordanted onto silk, so these colorants should not be considered to be inert even if they fade slowly on silk. Indigo blue on paper has been shown to fade rapidly in the presence of ozone in tests of both Western watercolor pigments and Japanese art (Whitmore et al. 1987; Whitmore and Cass 1988). In contrast, the Chinese colorants ban lan gen and da qing ye, when exposed to ozone, faded to an imperceptible degree on silk, and ban lan gen faded to a barely perceptible extent on paper. Although these colorants are derived from plants that can produce indigo, neither ban lan gen nor da qing ye as tested here have the blue color that traditionally comes to mind when one thinks of indigo blue (CI no. 73000). That the colorants are not chemically the same apparently explains the slower ozone fading rate of the ban lan gen and da qing ye samples tested here when compared to indigo blue. The very rapid initial reaction of certain of these colorants holds important implications for historical dyed silk (and possibly watercolor) samples unearthed during archaeological studies. Background ozone concentrations in rural atmospheres typically average about 40 ppb (0.04 ppm), a factor of only 10 lower than the concentrations used during the present experiments. Previous ozone fading experiments involving madder lakes show that the fading rate is directly proportional to the ozone concentration multiplied by the duration of exposure (Cass et al. 1991). Thus the initial fading seen during the first week of the present experiments would be expected to occur during about the first 10 weeks if newly excavated samples were exposed directly to outdoor ambient air. To avoid such damage, the samples can be placed into storage systems that do not allow air to circulate freely over the samples, or alternatively ozone can be removed from the storage and working environment by filtration through activated carbon or by careful management of building air exchange rates (Cass et al. 1991). 5 5. CONCLUSIONSExposure to atmospheric ozone at the concentrations seen in some urban atmospheres will lead to the fading of many traditional Chinese colorants that are derived from plant materials. Colorants applied as a thin wash on the surface of paper are particularly vulnerable, while silk samples dyed by traditional procedures also will fade but at a slower rate. Samples of the dyes jiang huang (turmeric), zi cao (gromwell), and zi ding cao (violet) applied on paper faded by ΔE > 10 when exposed to 0.40 ppm ozone at 25�C, 50% RH for 22 weeks. Nearly all other colorant samples on paper changed by ΔE > 2 over the course of the same experiment. Silk samples dyed with zi cao (gromwell), su mu (sappan wood), huang zhi zi (gardenia), zi ding cao (violet), and jianghuang (turmeric) also changed color by an amount ΔE > 2. The ozone concentration used in these experiments is at the upper end of the range of concentrations actually observed in urban atmospheres and is about 10-fold higher than the average ozone concentration present even in remote areas around the world. At these lower global background concentrations it would take 10 times longer (i.e., 220 weeks) of exposure to outdoor air to achieve the same degree of fading as observed in these experiments. However, since much of the color change measured in this work occurred rapidly during the first weeks of the experiment, we suggest that newly excavated dyed silk samples and samples containing Chinese natural organic colorants on other substrates should not be left exposed directly to the atmosphere (even in rural areas) for weeks at a time. Properly designed enclosures (storage boxes or cases) can scavenge ozone from the surrounding atmosphere and prevent its reentry into the storage system, thereby protecting the colored objects from premature fading due to ozone exposure. ACKNOWLEDGEMENTSThe silk and dried plant material samples used in this research were made available with the cooperation of Ms. Qian Xiaoping, Suzhou Silk Museum, Suzhou, China. REFERENCESBillmeyer, F. W., Jr., and M.Saltzman. 1981. Principles of color technology. 2d ed. New York: John Wiley & Sons. Campbell, G. G., G. G.Schurr, D. E.Slawikowski, and J. W.Spence. 1974. Assessing air pollution damage to coatings. Journal of Paint Technology46:59–71. Cass, G. R., W. W.Nazaroff, C.Tiller, and P. M.Whitmore. 1991. Protection of works of art from damage due to atmospheric ozone. Atmospheric Environment25A:441–51. De Wit, H. C.1965. Plants of the world, vol. 2. New York: E. P. Dutton & Co. Drisko, K., G. R.Cass, P. M.Whitmore, and J. R.Druzik. 1986. Fading of artists' pigments due to atmospheric ozone. In Wiener berichite �ber naturwissenschaft in der kunst, vol. 2–3, ed. A.Vendl, B.Pichler, and J.Weber. Vienna: Verlag ORAC. 66–87. Ehling, D., and S.Swart. 1996. The Chinese herbalist's handbook. Santa Fe, N.M.: High Mountain Press. Forbes, R. J.1987. Studies in ancient technology, vol. 4. Leiden and New York: E. J. Brill. Grieve, M.1971. A modern herbal. New York: Dover Publications. Grosjean, D., P. M.Whitmore, C. P.De Moor, G. R.Cass, and J. R.Druzik. 1987. Fading of alizarin and related artists' pigments by atmospheric ozone: Reaction products and mechanisms. Environmental Science and Technology21:635–43. Grosjean, D., P. M.Whitmore, and G. R.Cass. 1988a. Ozone fading of natural organic colorants: Mechanisms and products of reaction of ozone with indigos. Environmental Science and Technology22:292–98. Grosjean, D., P. M.Whitmore, C. P.De Moor, G. R.Cass, and J. R.Druzik. 1988b. Ozone fading of organic colorants: Products and mechanism of the reaction of ozone with curcumin. Environmental Science and Technology22:1357–61. GuptaD. B., and M. L.Gulrajani. 1996. The light fading mechanism of dyes derived from rhubarb extract. Journal of the Society of Dyers and Colourists112:269–72. Hayashi, K.1979. Chemical procedure for the determination of plant dyes in ancient Japanese textiles. In International symposium on the conservation and restoration of cultural property.Tokyo: National Research Institute of Cultural Properties. 39–50. Johnston-Feller, R., R. L.Feller, C. W.Bailie, and M.Curran. 1984. The kinetics of fading: Opaque films pigmented with alizarin lake and titanium dioxide. Journal of the American Institute for Conservation23:114–29. Kerr, N., M. A.Morris, and S. H.Zeronian. 1969. The effect of ozone and laundering on a vat-dyed cotton fabric. American Dyestuff Report58:34–36. Kuhn, D.1988. Science and civilisation in China—textile technology: Spinning and reeling, vol. 5, part 9. New York: Cambridge University Press. Li, S. Z. (1518–1593) 1981. Ben cao gang mu. (The great pharmacopoeia). Beijing: People's Health Publishing House. Liu, Y. C.1988. The essential book of traditional Chinese medicine. New York: Columbia University Press. Needham, J.1954. Science and civilisation in China. New York: Cambridge University Press. Needles, H. L., V.Cassman, and M. J.Collins. 1986. Mordanted, natural-dyed wool and silk fabrics. In Historic textiles and paper materials, ed. H. L.Needles. Advances in Chemistry series 212. Washington, D.C.: American Chemical Society. 199–210. Newton, R. G.1945. Mechanism of exposure-cracking of rubbers. Journal of Rubber Research14:27–39. Pratt, L. S.1947. The chemistry and physics of organic pigments. New York: John Wiley & Sons. Salvin, V. S.1969. Ozone fading of dyes. Textile Chemist and Colorist1:245–51. Scott, P.1993. The book of silk, London: Thames and Hudson. Seinfeld, J. H., and S. N.Pandis. 1998. Atmospheric chemistry and physics. New York: John Wiley & Sons. Shaver, C. L., G. R.Cass, and J. R.Druzik. 1983. Ozone and the deterioration of works of art. Environmental Science and Technology17:745–52. Society of Dyers and Colourists. 1971. Colour index, vol. 3, 3d ed.. Bradford, England: Society of Dyers and Colourists. Song, Y. X. (b. 1587) 1982. Tian Gong Kai Wu(The exploitation of the works of nature). Taibei: Times and Culture Publishing Co. Tang, J. J.1991. Analysis of plant dyes and study on ancient silk conservation. In Proceedings of the EEC China Workshop on Preservation of Cultural Heritages, Xian, Shaanxi, China. Naples, Italy: Teti. 317–33. Watt, J. C.1997. When silk was gold: Central Asian and Chinese textiles. New York: Metropolitan Museum of Art. Wee, Y. C.1992. An illustrated dictionary of Chinese medicinal herbs, Sebastopol, Calif.: CRCS Publications. Whitmore, P. M., G. R.Cass, and J. R.Druzik. 1987. Ozone fading of traditional natural organic colorants on paper. Journal of the American Institute for Conservation26:45–58. Whitmore, P. M., and G. R.Cass. 1988. The ozone fading of traditional Japanese colorants. Studies in Conservation33:29–40. Wu, S. S., and Z. B.Tian. 1986. Zhong guo yin ran shi(Chinese history of dyeing and weaving). Shanghai: People's Publishing House. Zhao, K. H.1985. Zhong guo gu dai hua xue shi yian jiu(Studies in the Chinese history of ancient chemistry). Beijing: Peking University Press. AUTHOR INFORMATIONYUN YE is a Ph.D. candidate in the Materials Science Department at the California Institute of Technology. She received a B.S. in materials science from the Tsinghua University at Beijing, China, in 1995, and an M.S. in materials science from California Institute of Technology in 1997. Address: Mail code 138–78, Materials Science Department, California Institute of Technology, Pasadena, Calif. 91125 LYNN G. SALMON received a B.S. in materials science from the Massachusetts Institute of Technology and an M.S. in materials science from UCLA. Since 1986 she has been a research engineer at the California Institute of Technology, where she has conducted numerous studies of airborne pollutants in museums, national parks, and archaeological sites around the world. Address: Mail Code 138–78, Environmental Engineering Science Department, California Institute of Technology, Pasadena, Calif. 91125 GLEN R. CASS received his Ph.D. from the California Institute of Technology in 1978. At the time of this work he was a professor of environmental engineering and mechanical engineering at Caltech. He is presently chair of the School of Earth and Atmospheric Sciences at the Georgia Institute of Technology, Atlanta. Current research interests center on control of air pollution problems, including the problem of protection of works of art from damage due to air pollution. Address: School of Earth and Atmospheric Sciences, Georgia Institute of Technology, 221 Bobby Dodd Way, Atlanta, Ga. 30332 Received for review May 17, 1999. Revised manuscript received December 1, 1999. Accepted for publication March 14, 2000.
 Section Index Section Index |