CYCLODODECANE: TECHNICAL NOTE ON SOME USES IN PAPER AND OBJECTS CONSERVATIONIRENE BR�CKLE, JONATHAN THORNTON, KIMBERLY NICHOLS, & GERRI STRICKLER
ABSTRACT—Cyclododecane, a volatile cyclic alkane (C12H24), was recently introduced to the conservation field as a temporary consolidant or masking material with suggested uses in paintings, textile and architectural conservation. In the present study, the waxlike hydrocarbon material was tested for its potential use as a temporary fixative for water-sensitive media on paper using an adaptation of a commercial wax-melting tool. Cyclododecane offered considerable, although not always complete, protection during aqueous treatments performed on media with different degrees of water sensitivity. The hydrocarbon was further investigated as a barrier film for taking molds from objects with porous surfaces that are easily contaminated by residues of the silicone mold materials commonly used. In combination with gum arabic or methylcellulose, this barrier system virtually eliminated the penetration of the silicone oils into the surface of modern flowerpot shards and limestone samples. In both of these applications, cyclododecane sublimed completely from the substrates after treatment. These findings suggest that other improvements of current treatments in different conservation specialties may be realized through the use of cyclododecane. TITRE—Le Cyclodod�cane: Notes Techniques sur Certains Usages dans les Domaines de Restauration du Papier et des Objets. R�SUM�—Le cyclodod�cane, un alcyne cyclique volatil (C12H24) a r�cemment �t� introduit dans le domaine de la restauration, comme consolidant temporaire et couche protectrice dans le traitement des tableaux, des textiles et des �l�ments architecturaux. Cet hydrocarbure semblable � une cire a fait l'objet de tests pour d�terminer son potentiel en tant que fixatif temporaire pour des liants solubles � l'eau sur des oeuvres en papier, ceci en utilisant une version adapt�e d'un outil pour travailler la cire � chaud. Lors de traitements aqueux, le cyclododecane prot�gea de facon consid�rable, quoique pas toujours compl�te, un certain nombre de liants aux solubilit�s variables dans l'eau. L'hydrocarbure fit aussi l'objet de tests en tant que film protecteur lors du moulage d'objects en mati�re poreuse, objets qui sont facilement contamin� par les r�sidus des produits de moulage fr�quemment utilis�s � base de silicone. Lorsqu' utilis� en combinaison avec de la gomme arabique ou du m�thyle de cellulose, ce film protecteur emp�cha presque compl�tement la p�n�tration des huiles de silicone dans des fragments de poterie moderne et des �chantillons de calcaire. Apr�s traitement, dans les deux types d'application, la sublimation du cyclodod�cane des surfaces trait�es fut compl�te. Ces r�sultats sugg�rent que d'autres m�thodes actuelles de traitement dans divers domaines de la restauration pourraient �tre am�lior�es par l'utilisation du cyclodod�cane. TITULO—Ciclododecano: nota t�cnica sobre algunos usos en la conservaci�n de papel y objetos. RESUMEN—El Ciclododecano, un alcano c�clico vol�til (C12H24), fue introducido recientemente en el campo de la conservaci�n como un consolidante temporal o como un material protector cuyo uso es sugerido en la conservaci�n de pinturas, textiles y arquitectura. En el presente estudio fue examinado el posible uso de este hidrocarburo, similar a la cera, como un fijador temporal para medios sensibles al agua utilizados sobre papel adaptando para ello un equipo comercial para derretir ceras. El Ciclododecano ofreci� considerable protecci�n, aunque no completa en todos los casos, durante tratamientos acuosos realizados en medios con diferente grado de sensibilidad 1 INTRODUCTIONIn 1995, Hans Michael Hangleiter, Elisabeth J�gers, and Erhard J�gers published an article on a material that they proposed might be important for different conservation specialties. The authors described their search for a binding agent that could be applied as a temporary consolidant during treatments. The ideal agent would have good film-forming properties, low melting point, insolubility in water, solubility in organic solvents, and little or no toxicity. Most important, its application would be easily reversible. Their investigation led them to a series of alicyclic hydrocarbons, compounds of a waxlike consistency that can be melted at temperatures ranging from 35 to 65�C. These materials have as their most important common characteristic the ability to sublime, i.e., to volatilize slowly from a solid directly into a gas under ordinary room-temperature conditions. This characteristic eliminates the need for other chemical or physical means of removal necessary with most other consolidants. Four similar substances with the abovenoted characteristics, all differing mainly in their melting point temperatures and also in the rate or speed of their sublimation, were discussed by Hangleiter and his coauthors. These are camphene (mp 45–46�C); camphene-tricyclene, a mixture of two hydrocarbon types (mp 35�C); menthol (mp 31–35�C); and cyclododecane (mp 58–61�C). The hydrocarbons, termed by the authors “volatile binders,” can be applied in molten state or can be dissolved in nonpolar solvents such as saturated, aromatic, or halogenated hydrocarbons or petroleum ethers. The sublimation of the binders can be retarded by choosing one with a high melting point, applying it in a molten state, keeping the treated object in a contained environment, and protecting the treated object from exposure to elevated temperatures. Since 1995, several potential uses of volatile binders for situations where temporary consolidation and impregnation of fragile materials or objects are desirable have been proposed and/or tested in treatment:
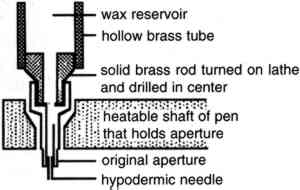
These authors did not explore all the compatibilities of the hydrocarbon substances with other materials. They pointed out, however, that the cycloalkanes might interact with nonpolar constituents of paint films or varnishes. Gudrun Hiby (1997), for example, found that cyclododecane altered the appearance of a new acrylic paint film. Nicole Riedl and Georg Hilbert (1998) warned that residues of volatile hydrocarbons may remain for extended periods in the interior of mortar substrates. The present study continues the exploratory investigations. We chose cyclododecane for our testing because it sublimes slowly, is chemically stable, has no known toxicity, and at present is more readily available than other cycloalkanes. It has been used in the chemical industry as an additive for fragrances and synthetic waxes (DuPont Nylon Intermediates 1998). The chemical properties of cyclododecane have been the subject of numerous scientific studies (e.g., Kolossv�ry and Guida 1993) and are beyond the scope of this article. Of importance for conservation are the physical characteristics of this substance (Hangleiter et al. 1995; Hiby 1997). Cyclododecane has the low solubility parameter and surface energy values comparable to a hydrocarbon wax. It forms a relatively hard, waxy-looking solid, and it has a boiling point of 243�C and a melting point of 58–61�C. The melted material solidifies quickly upon cooling. It is soluble in nonpolar organic solvents but insoluble in acetone, ethanol, or water, and it is impermeable to acids and bases. It volatilizes slowly; Hans Michael Hangleiter et al. (1995) observed that a solid film sublimed at a rate of 0.03 mm per 24 hours. Our tests showed that a film of ca. 0.08 mm thickness brushed out on glass started to disappear within one day if kept at 20�C and disappeared completely within three days. At 38�C, a film of the same thickness disappeared completely within one day. We investigated aspects of the application of cyclododecane in two areas of conservation treatment: (1) the isolation of water-sensitive media on paper objects that will undergo aqueous treatment and (2) the protection of the substrate of porous objects that are to be cast for replication using a silicone mold. Mention of related subjects appeared in Geller (1997) and Hangleiter (1998b). 2 CYCLODODECANE AS A TEMPORARY FIXATIVE FOR WATER-SENSITIVE MEDIAThe aqueous treatment of paper has always been limited to some extent by the water-sensitivity of media associated with it. A number of treatment options have been developed that control moisture access to water-sensitive media during washing, among them blotter- or float-washing and use of a suction table. Only a few treatment choices allow the conservator to isolate or fix water-sensitive media for better protection during aqueous treatments. Several fixatives for the temporary isolation or impregnation of water-sensitive media have been used over the years. The most common is probably Paraloid B-72, although waxes and BEVA have also been employed (Rodgers [1988] 1994). In fine art paper conservation, the application of temporary fixatives is considered only with hesitancy. If used, they are commonly employed to protect during treatment small features of an object, such as an artist's signature or estate stamp. These features are often sensitive to aqueous treatment yet are difficult to test for sensitivity and allow for little risk-taking during treatment because, despite their small size, they often constitute a prominent 2.1 APPLICATION METHODA solid film of cyclododecane is formed either by cooling the molten material or by allowing an organic solvent to evaporate from a saturated solution of cyclododecane. The film conforms to the topography of the substrate to which it is applied (Riedl and Hilbert 1998). Its characteristics may vary depending on which of the two application methods is chosen (Hiby 1997), the porosity of the substrate, and the rate of film formation. In our tests, carried out at 45% RH, 21�C, a film formed after the evaporation of petroleum ether (boiling range 35–60�C) from a saturated cyclododecane solution brushed on an unheated glass slide was composed of large crystals, whereas cyclododecane applied in molten condition to a glass slide contained smaller crystals (fig. 1). Preliminary trials on a test object (“DRAFT” stamp, see sec. 2.2.1) showed that molten cyclododecane provided a better barrier than a solvent-based film for the protection of water-sensitive media. Hiby (1997) had melted cyclododecane using a heated spatula. To refine our treatment so that it would allow the isolation of narrow ink lines or other small-scale features on paper, we slightly modified an electric wax-melting pen (Kistka) that can be heated to 100�C. The wax reservoir of the pen was enlarged by a latheturned attachment fashioned from brass rod and brass tubing that were soldered together. This modification allowed longer working times between refills of cyclododecane. Because the apertures supplied with the instrument were too wide to allow a precise application of cyclododecane, the no. 2 aperture was fitted with a section of a 27 gauge, 1/2 in. long hypodermic
2.2 TREATMENTSCyclododecane was tested on a modern office stamp and three historical samples of iron gall ink. The modern office stamp underwent a selection of test treatments that served as a standard in this project. An expendable letter address written in iron gall ink underwent a mock washing treatment. Two artifacts that carried iron gall ink inscriptions were treated in case studies. The cyclododecane film was allowed to sublime before the objects were visually examined after treatment for the degree of bleeding observed. 2.2.1 “DRAFT” StampPaper and media: A modern, red-colored commercial office stamp that spells out the word “DRAFT” was stamped evenly on late-19th-century book papers. The brightly colored stamp pad ink was chosen as a test material because, being a molecularly dispersed colorant, it is very water soluble and could easily be read for signs of bleeding. The two types of paper were of similar rag fiber content, comparable thickness and smooth texture. One paper was sized, presumably with alum-rosin size. It tested positive for alum using the Aluminon test and repelled a water droplet placed on the paper surface. The other paper was unsized. Treatment procedure: Cyclododecane was applied with the wax-melting pen to the individual letters of one set of stamps, slightly overlapping the edges of each letter. The material impregnated the paper completely in the areas of application. The stamps underwent treatment directly after the application of cyclododecane. One other set of stamps was treated in unfixed condition, and another set was fixed with a 15% solution of B-72 (1:1 toluene:xylene). B-72 was brushed twice on the stamps, slightly overlapping the edges of the letters. The fixed area appeared visually well impregnated with B-72, judging by the transparency of the paper. Four different aqueous treatments were performed on the three sample sets (deionized water, pH 8.5 with calcium hydroxide): humidification, float washing, immersion washing, and blotter washing. Each treatment was allowed to continue for different lengths of time: 5, 15, 30, and 60 minutes. After treatment, the samples were allowed to air-dry on a screen. They were then visually compared with the untreated controls. Results: Cyclododecane compared favorably with B-72. Both materials effected substantial protection of the “DRAFT” stamp (table 1). With increasing duration of the treatment, however, both fixatives failed to protect the stamp pad ink completely from water penetration. The samples treated by immersion showed, as expected, the greatest degree of lateral bleeding of the ink. The unsized paper was more susceptible to the penetration of water than the sized paper, and the stamps on the unsized paper therefore suffered more damage than those on the sized paper. This difference was especially noticeable in the treatments that allowed greatest water contact with the samples, float washing and immersion. TABLE 1. DEGREE OF INK BLEEDING AFTER AQUEOUS TREATMENTS 2.2.2 Iron Gall Ink Letter AddressPaper and media: The address, dated 1848, was written on a sheet of handmade rag paper that had been folded into an envelope. The paper was thin and gelatin-alum-sized. It tested positive for gelatin using the Biuret test and positive for alum using the Aluminon test. The ink had slightly degraded the paper, causing local light brown and dark brown stains on the verso. The paper was otherwise in good condition and showed no signs of embrittlement in the areas of ink application. During water droplet testing, slight lateral migration of colored ink components occurred ca. 3 minutes after water was applied to the ink surface.
Treatment procedure: The letter address was impregnated with cyclododecane, which was allowed to extend slightly beyond the ink lines and penetrate the paper. The paper was float washed for 20 minutes in a water bath (pH 8.5
Results: Cyclododecane did not protect the ink inscription sufficiently, for several possible reasons. First, the thin, gelatin-sized paper expanded ca. 3% in the cross-grain direction when moistened. (The “DRAFT” stamp paper supports expanded only ca. 1% in the crossgrain direction.) A brittle cyclododecane film does not undergo the same expansion as the dampened paper sheet. During treatment, the fixative film may have developed fine crack patterns or openings invisible to the naked eye that would have allowed moisture to penetrate the fixed area. Further, the cyclododecane film inevitably was slightly flexed during the treatment procedure, a situation that may have caused cracks in the film, especially since the material was applied across a relatively wide area of the paper spanning the lengths of individual words (ca. 4 cm) (fig. 3). Third, the paper contained long fibers that extended from the unfixed into the fixed areas. Moisture was able to travel along or within these fibers and penetrate
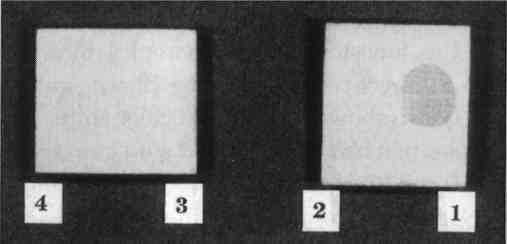
2.2.3 Case StudiesChromolithograph: The advertisement was printed around 1865 on smooth, machinemade, uncoated bast fiber paper, most likely unsized or only weakly alum-rosin-sized. The paper absorbed water quickly and tested negative for alum using the Aluminon Test. The print was severely stained by tide lines and general discoloration. In a cartouche in the bottom center, the print was signed in iron gall ink, “E. C. Pasco, Agent.” The ink lines were crisp and dense along the vertical pen strokes. Although the ink did not show signs of sensitivity during a water droplet test (performed as in 2.2.2), the clarity of the inscription would have most certainly suffered during the extended aqueous treatment required to reduce the severe paper discoloration. Cyclododecane was applied before aqueous treatment. The ink lines showed no loss of crispness after ca. 80 minutes of float washing and immersion washing (fig. 5).
Chalk drawing: The drawing, made in 1912 in Palermo, Italy, had been fixed with a natural resin varnish (the varnish fluoresced yellowish under UV illumination). The machine-made drawing paper (watermark. “A. BINDA. E.C.T.”) was thick and slightly textured, and it may have been weakly sized with alum-rosin sizing. It tested slightly positive for alum using the Aluminon Test, tested negative for gelatin using the Biuret Test, and absorbed a water droplet only slowly. The drawing was signed in the lower margin by the artist in iron gall ink, “F. Balistrieri, 1912.” The signature did not show signs of sensitivity during a water droplet test (performed as in 2.2.2), but it was feared that its crisp appearance might be compromised during aqueous treatment. The objective of the treatment was to lighten the severely discolored blank paper margins. The application of cyclododecane to the signature allowed the paper margins to be covered by water during
2.3 RESULTSThe tests carried out in this brief study only partially replicated actual treatment situations. Under ordinary circumstances, the treatment of the highly water-sensitive “DRAFT” stamp would have not included such aggressive steps as, for example, immersion washing. Treatments of the stamp and the letter address showed that there are limitations to the efficacy of cyclododecane impregnation that must be considered when designing treatments using this material. Very water-sensitive media, especially if they are situated on unsized paper, may not be protected sufficiently by cyclododecane (and may, for that matter, be difficult to isolate with other types of fixatives). The treatment results of the chromolithograph and the chalk drawing suggest, however, that there are cases where media can be successfully protected by cyclododecane during aqueous treatment. Although neither of these iron gall inks showed any water sensitivity during pretreatment testing, it is very likely that they would have been affected if washed without the application of a protective coating. It seems, therefore, that cyclododecane may be suitable especially when media are suspected to show some, but not extreme, water sensitivity during aqueous treatment and when some measure of protection seems advisable. The effectiveness of cyclododecane as a fixative depends to some extent on the type and condition of the paper to which it is applied. Thick, dimensionally stable papers (such as the chalk drawing in 2.2.3) may be less likely to cause water penetration into the cyclododecane layer during treatment than thin papers and those that will expand greatly upon wetting (such as the handmade paper in 2.2.2). Papers made of strongly beaten fibers (such as the chromolithograph in 2.2.3) may be more successfully isolated with cyclododecane than papers that contain long, undamaged fibers (such as those in 2.2.2). Two measures may improve the impregnation of media with cyclododecane: applying the material from both sides of the paper and slightly warming the paper area during application. Cyclododecane appeared to sublime completely from the substrates after treatment. Selected samples were washed after the disappearance of cyclododecane to test whether the presence of the material had changed the wetting properties of the paper. None of the samples showed altered water absorption patterns in the areas where cyclododecane had been applied. Cyclododecane certainly cannot be regarded as a solution to all potential problems associated with the aqueous treatment of water-sensitive media on paper. The results of this short study indicate, however, that the material provides protection to media that can, under certain conditions, be equal to that offered by B-72. When compared to this and other fixatives, cyclododecane offers the following advantages:
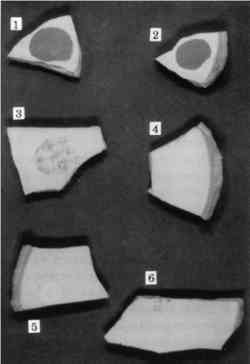
3 CYCLODODECANE AS AN ISOLATING LAYER TO PREVENT STAINING OF POROUS MATERIALS DURING MOLDMAKINGMolding and casting of museum objects is carried out frequently and for a variety of reasons, among them to restore missing parts during restoration; to supply replicas of important objects to other institutions and researchers; to provide expendable reproductions for display and handling in interactive and “living history” exhibits; and to create authentic replicas for sale in museum shops. Conservators are often called upon to make the molds or to oversee and make material recommendations to mold makers. So-called silicone rubbers of the RTV-2 type (room-temperature vulcanizing polymers that cure after the mixing of two separate components) have been by far the most common mold-making materials for these purposes. They pick up very fine detail, cure to a strong flexible mass without shrinkage, release well from both the objects and the materials used to make positive casts, and have good permanence as finished molds. A drawback to their use, however, is the staining that can occur on porous surfaces due to the migration of uncured low-molecular-weight “silicone oils” before the rubber has cured, a problem noticed by many conservators and briefly noted in the literature (Larsen 1981). The researcher who addressed this problem most extensively reported that the least damage was caused by the use of barrier films and fast-curing rubbers but all caused some damage (Maish 1994). Since barrier films themselves posed removal problems and did not serve as effective barriers unless they were thick enough to obscure detail, not truly satisfactory solution to this problem then existed. Cyclododecane suggested itself to the authors as a useful barrier to prevent the staining caused by uncured silicone rubber. If it truly did sublime completely, it would not need to be removed with solvents after the molding procedure. The likelihood of leaving behind residual barrier materials would be reduced, as would the chance of inadvertently “cleaning” the object, as sometimes happens due to the impregnation of surface deposits with the rubber and the subsequent removal of these deposits with the mold. Cyclododecane was tested as a barrier material on shards of modern terracotta flower pots and tiles of oolitic Indiana limestone. These examples were chosen as typical of the types of surfaces that would be most likely to stain during molding. 3.1 APPLICATION OF CYCLODODECANEWhile cyclododecane can be deposited out of organic solvent solutions, our experience, as previously mentioned, indicated that this procedure resulted in the formation of large, needlelike crystals that would not form a consistent barrier film of sufficient homogeneity. While other authors reported success in solvent-borne application, our experiments indicated that a consistent compact layer could be For our molding tests, we chose a silicone rubber (Dow Corning 3110) that has been used extensively in conservation. In keeping with the recommendations of Jeffrey Maish (1994) for least staining, we chose the fast catalyst (no. 4, a tin catalyst). It became immediately apparent that cyclododecane alone would not function as a barrier to silicone oils. It is a nonpolar substance and is probably either penetrated or partially dissolved by the nonpolar silicone oils, and staining was as severe with the cyclododecane film as without it. While this development was initially discouraging, we next tried the addition of thin isolation films of polar materials on top of the cyclododecane. This procedure seemed to provide a promising solution to the problem. When used in this way, the cyclododecane would fill the interstitial voids and prevent penetration of the functional isolation film. This isolation film could thus be very thin and would be easy to remove prior to the subsequent sublimation of the cyclododecane. 3.2 TESTING OF BARRIER SYSTEMSWe chose four water-soluble polar isolation materials for further testing in combination with cyclododecane: gum arabic, polyvinyl alcohol, Aquazol (Poly (2-ethyl-2-oxazoline)) (Chiu and Fairchock 1986; Wolbers et al. 1998), and methylcellulose. These materials were chosen because they would not mix with either the cyclododecane or the silicone rubber and would be readily removable with water. Previous experiments had indicated that none of these materials, when used on their own, provided adequate protection because they were not completely removable, did not prevent penetration of silicone oil, or both. Low-molecular-weight versions of the synthetic polymers were chosen because it was felt that they would provide the best barriers. Concentrations were chosen that would be fluid enough to be easily applied by brush and dry to a thin film that would not affect surface detail. These isolation films were applied on top of surfaces that had been previously impregnated with cyclododecane, and the mold rubber was cast onto the surfaces immediately. After removal of the mold, the isolation film was removed with water, and the cyclododecane was allowed to sublime in a warmed light box (38�C) that accelerated the sublimation process. 3.3 RESULTSResults of our tests are summarized in table 2. The use of gum arabic and methylcellulose as isolating layers between the cyclododecane impregnation and the mold material gave visually acceptable results on both the terracotta and limestone samples and provided highly detailed molds of surface textures (figs. 7 and 8). As expected, the unprotected terracotta shard (no. 1) that had been in direct contact with silicone rubber showed a significant staining. The shard that had been impregnated with cyclododecane but had not received an isolating layer before molding (no. 2) was also significantly stained. An isolating film of Aquazol over the cyclododecane (no. 3) did not prevent the penetration of silicone oils into the terracotta, and it proved difficult to remove. When used in conjunction with cyclododecane, methylcellulose (no. 5) and gum arabic (no. 6) showed only barely detectable color shifts. Of
TABLE 2. SUMMARY OF RESULTS OF MOLDING TEST TREATMENTS The limestone control samples that had been in direct contact with the silicone rubber (no. 1) also showed significant color shifts. The samples that had been isolated with gum arabic (no. 4) exhibited slightly darker speckles in the stone surface. Compared with gum arabic and polyvinyl alcohol (no. 2), methylcellulose (no. 3) performed slightly better as an isolating layer when used in conjunction with cyclododecane. Overall, the results of this study are promising. Protecting the surfaces of porous substrates with a cyclododecane barrier film proved highly effective if an additional isolating layer of methylcellulose or gum arabic was used. Although even the most successful treatments caused very slight darkening of the substrates, this color change was virtually imperceptible and in the experience of one of the authors (Thornton) is an improvement over the results that can be obtained by other isolating strategies. Further, the authors believe that the slight darkening experienced by the successfully treated samples could be potentially eliminated if a second or third layer of the isolating film was applied or a single layer of a more concentrated solution were applied to form a denser isolating film. It is unlikely that either would interfere with the fidelity of the cast except where extremely fine detail is required, for example in the preparation of casts for electron microscopy. While there is hope for further refinement of method, initial results are very promising. 4 SUMMARY AND SUGGESTED FURTHER RESEARCHAs suggested in the conservation literature, cyclododecane appears to have potential for a variety of applications in different conservation specialties. The authors explored a few of these uses in paper and objects conservation. In both cases, they found that cyclododecane applied molten was more useful than when applied dissolved in an organic solvent, though there may also be potential for refinement of solvent application methods that also may be aided by solvent-saturated atmospheres (Hansen et al. 1993). While the test treatment results were not completely flawless in all cases, the purpose of the tests was to establish a range of possible practical applications of the material that will improve existing treatment methods. Future studies may consider a closer look at the process of cyclododecane sublimation from different substrates. In paper conservation, future work may elucidate the way in which cyclododecane interacts with a greater variety of media and papers than were investigated here. It will be important, for example, to understand how cyclododecane interacts with deteriorated iron gall ink that is susceptible to flaking and/or is associated with paper embrittlement (van Gulik and Kersten-Pampiglione 1994; van der Windt 1997). Different cycloalkanes may be tested for comparison. Potential microscopic changes in the physical structure of the cyclododecane film during treatment should be checked under high magnification, such as scanning electron microscopy. Other uses of cyclododecane not yet suggested in the literature pertain to diverse objects conservation problems. Cyclododecane might be used to prevent the imbedding of white fill materials into adjacent rough and porous surfaces, a phenomenon generally referred to as “ghosting.” It could be used to create detachable plaster and polymer fills in ceramics and glass conservation, a procedure previously accomplished with other isolating resins. Porous corrosion products could be impregnated with cyclododecane to allow the localized and topical use of acidic or basic cleaning reagents. It could be used as a masking material during aesthetic compensation of missing painted detail, particularly when using an airbrush. REFERENCES
Chiu, T. T., and W. J., Fairchock. 1986. Poly (2-ethyl-2oxazoline): A new water- and organic-soluble adhesive. In Water-soluble polymers: Beauty with performance, ed.J. E.Glass. DuPont Nylon Intermediates. 1998. Personal communication. B.M.P. 24, P.O. Box 80024, Wilmington, DE 19880-0024. (800) 231-0998. Hangleiter, H. M.1998a. Erfahrungen mit fl�chtigen Bindemitteln. Part 1. Restauro5: 314–19. Hangleiter, H. M.1998b. Erfahrungen mit fl�chtigen Bindemitteln. Part 2. Restauro7: 468–73. Hangleiter, H. M., E.J�gers, and E.J�gers. 1995. Fl�chtige Bindemittel. Zeitschrift f�r Kunst-technologie und Konservierung2: 385–92. Hansen, E. F., R., Lowinger, and E., Sadoff. 1993. Consolidation of porous paint in a vapor-saturated atmosphere: A technique for minimizing changes in the appearance of powdering, matte paint. Journal of the American Institute for Conservation32: 1–14. Hiby, G.1997. Das fl�chtige Bindemittel Cyclododecan. Restauro2: 96–103. Kolossv�ry, I., and W. C., Guida. 1993. Comprehensive conformational analysis of the four- to twelve-membered ring cycloalkanes: Identification of the complete set of interconversion pathways on the MM2 potential energy hypersurface. Journal of the American Chemical Society115: 2107–19. Larsen, E. B.1981. Molding and casting of museum objects. Copenhagen: Konservatorskolen, Det Kongelige Danske Kunstakademi. Maish, J. P.1994. Silicon rubber staining of terracotta surfaces. Studies in Conservation39: 250–56. Riedl, N., and G.Hilbert. 1998. Cyclododecan im Putzgef�age. Restauro7: 494–99. Rodgers, S. M., [1988] 1994. Consolidation, fixing, facing. In Paper conservation catalog. Washington, D.C.: American Institute for Conservation. 1–20. van derWindt, H., ed.1997. Proceedings, European Workshop on Iron-Gall Ink Corrosion. Rotterdam: Museum Boijmans Van Beuningen. vanGulik, R. and N. E., Kersten-Pampiglione. 1994. A closer look at iron gall ink burn. Restaurator15: 173–87. Wolbers, R., M., McGinn, and D., Duerbeck. 1998. Poly(2-ethyl-2-oxazoline): A new conservation consolidant. In Painted wood: History and conservation, ed.V.Dorge and F.C.Howlett. Los Angeles: Getty Conservation Institute. 514–27. SOURCES OF MATERIALSParaloid B-72Rohm & Haas, Independence Mall West, Philadelphia, PA 19105 CyclododecaneKremer Pigments, Inc., 228 Elizabeth St., New York, NY 10012 “DRAFT” stampSanford POM� Stamper, #00224, manufactured in Japan Electric wax melting pen (Kistka)Used for decorating Ukranian Easter, eggs; order aperture nos. 0, 1, 2, Delta Ukranian Enterprise, 2242 West Chicago Ave., Chicago, IL 60622 gum arabicDow Chemical Co., Midland, MI, hypodermic needle (27 G 1/2) Dow Chemical Co., Midland, MI polyvinyl alcohol (low molecular weight)Conservator's Emporium, 100 Standing Rock Circle, Reno, NV 89511 AquazolConservator's Emporium, 100 Standing Rock Circle, Reno, NV 89511 AUTHOR INFORMATIONIRENE BR�CKLE holds an M.A. in art history from the State University of New York at Buffalo and is pursuing a Ph.D. in comparative literature there. She is an associate professor in the Art Conservation Department at the State University College at Buffalo, where she has taught since 1994. She received her paper conservation and bookbinding training in Germany. Address: Art Conservation Department, RH 230, Buffalo State College, 1300 Elmwood Ave., Buffalo, N.Y. 14222. brueckle@buffnet.net JONATHAN THORNTON received his M.A. and certificate of advanced study from the State University College at Oneonta, New York (Cooperstown Graduate Program in Conservation) after an earlier career as an independent craftsman. He is a professor in the Art Conservation Department of the State University College at Buffalo, where he has taught the conservation of objects since 1980. Address as for Br�ckle. KIMBERLY NICHOLS is a third-year student in paper conservation in the Art Conservation Department, State University College at Buffalo. She received her B.A. from Newcomb College, Tulane University, in art history. She worked at the Art Institute of Chicago, at Graphic Conservation in Chicago, and at the Library of Congress. She is spending her third-year internship at the California Palace of the Legion of Honor, Fine Arts Museums of San Francisco. Address as for Br�ckle. GERRI STRICKLER is a third-year student in objects conservation in the Art Conservation Department, State University College at Buffalo. She received her B.A. from the University of Delaware in art conservation and worked at the Los Angeles County Museum of Art and the Museum of New Mexico, Santa Fe. She is spending her third-year internship at the Gerald R. Ford Conservation Center in Omaha, Nebraska. Address as for Br�ckle.
 Section Index Section Index |