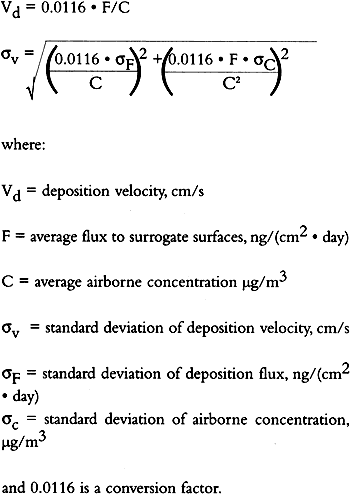VERTICAL GRADIENTS OF POLLUTANT CONCENTRATIONS AND DEPOSITION FLUXES ON A TALL LIMESTONE BUILDINGVICKEN ETYMEZIAN, CLIFF I. DAVIDSON, SUSAN FINGER, MARY F. STRIEGEL, NOEMI BARABAS, & JUDITH C. CHOW
ABSTRACT—The role of air pollutants in the soiling of a limestone building was investigated by measuring pollutant airborne concentrations and deposition at different heights at the Cathedral of Learning in Pittsburgh, Pennsylvania. Airborne concentrations of sulfate particles (SO42−), carbon particles, sulfur dioxide gas (SO2), and total NO3− (particles + HNO3) were measured simultaneously on the 5th floor, 16th floor, and roof (42d floor), while laser particle counts of >0.5 μm and >5 μm particles were obtained on the 5th and 16th floors. SO2 deposition fluxes to wall-mounted surrogate surfaces were measured at a total of nine locations on the 5th and 16th floors. Measurements were conducted during four 4-week time periods over one year. Results showed that airborne concentrations of the chemical species were invariant with height. Airborne number concentrations of >0.5 μm particles corroborated this result. Although not reflected in the chemical data, measured number concentrations of >5 μm particles on the 16th floor were on average 30% greater than those on the 5th floor. The spatially averaged highest and lowest deposition velocities of SO2 (1.0 cm/s and 0.6 cm/s) never differed by more than a factor of two for the different time periods. The relative differences in deposition velocities from one location to another were consistent throughout all of the sampling experiments. The 16th floor deposition velocities were greater than those on the 5th floor due, at least in part, to the fact that sampling locations on the 16th floor were more exposed to wind. The absence of gradients suggests that soiling patterns on the cathedral are determined by the competing processes of pollutant deposition and rain washing. This hypothesis is supported by comparing soiling patterns on the cathedral from the 1930s with recent patterns: Archival photographs show much greater amounts of soiling, consistent with the greater air pollution levels that existed then. Results of this study can assist in designing cleaning and treatment protocols for other buildings with similar geometry in similar environments. TITRE—Gradient vertical de la concentration des polluants et de l'�coulement des d�p�ts sur un haut �difice en calcaire. R�SUM�—Le r�le qu'ont les polluants dans la production de d�p�ts de salet� sur les �difices en calcaire a �t� �tudi� dans le cas de la Cathedral of Learning, � Pittsburg, en Pennsylvanie, en mesurant les concentrations des polluants dans l'air et les d�p�ts de salet� correspondant � la m�me hauteur de l'�difice. Les concentrations a�riennes de gaz SO2, de particules de SO42− et de carbone, ainsi que de NO3 sous forme de particules et de HNO3 ont �t� mesur�es simultan�ment � la hauteur des cinqui�me, seizi�me et quarante-deuxi�me �tages (toit) de la cath�drale. Le compte au laser des particules mesurant plus de 0,5 microns et plus de 5 microns fut obtenu seulement pour les cinqui�me et seizi�me �tages. Les �coulements de d�p�ts de SO2 sur des surfaces de contr�le install�es contre les murs furent mesur�s � neuf endroits diff�rents aux cinqui�me et seizi�me �tages. Les mesures furent effectu�es pendant quatre p�riodes de temps, chacune d'une dur�e de quatre semaines, durant le courant d'une ann�e. Les r�sultats, corrobor�s par les donn�es relatives aux particules de plus de 0,5 microns, d�montrent que les concentrations a�riennes des diff�rentes esp�ces chimiques ne varient pas en fonction de la hauteur. Le nombre de particules de plus de 5 microns �taient en moyenne 30% plus �lev� au niveau du seizi�me �tage par rapport � celui du cinqui�me �tage, quoique l'analyse chimique ne refl�te pas ce r�sultat. La moyenne spatiale des extr�mes des vitesses de d�position du SO2 (1,0 cm/s et 0,6 cm/s) n'a jamais vari�e au-del� d'un facteur de deux, lors des diff�rentes p�riodes de temps. Les diff�rences relatives dans les vitesses de d�position entre les diff�rents endroits mesur�s ont �t� conformes pendant toute la dur�e de l'�chantillonnage. Les vitesses de d�position �taient plus �lev�es au seizi�me qu'au cinqui�me �tage: ceci �tait partiellement d� au fait que les endroits mesur�s �taient plus expos�s au vent. L'absence de gradients sugg�re que les d�p�ts de salet�s sur les murs de la cath�drale se font en fonction de deux processus qui s'opposent: le d�p�t des polluants, et l'effet nettoyant des pluies. Une comparaison des d�p�ts de salet�s produits lors des ann�es trente et ceux produits aujourd'hui semble confirmer cette hypoth�se. Des photographies d'archives montrent qu'il y avait beaucoup plus de d�p�ts, ce qui s'explique par le plus haut taux de pollution de l'�poque. Les r�sultats de cette �tude peuvent aider � concevoir des porotocoles de nettoyage et de traitement pour des �difices g�om�triquement semblables se trouvant dans un environnement similaire. TITULO—Gradientes verticales de concentraci�n de contaminantes y flujos de sedimentaci�n en un edificio alto de piedra caliza. RESUMEN—El rol de los contaminantes atmosf�ricos en la adquisici�n de suciedad de un edificio de piedra caliza fue investigado midiendo la concentraci�n y la sedimentaci�n de contaminantes en el aire a diferentes alturas del edificio “Cathedral of Learning” en Pittsburgh, Pennsilvania, EE. UU. Las concentraciones atmosf�ricas de part�culas de sulfato (SO42−), part�culas de carb�n, gas sulfuro (SO2) y la cantidad total de NO3 (part�culas + HNO3) fueron medidas simult�neamente en los pisos 5, 10 y 6 y en el techo (piso 42), a la vez que se obtuvieron c�mputos l�ser de part�culas de > 0,5μm y >5μm en los pisos 5 y 16. Los flujos de sedimentaci�n de SO2 en superficies sustitutas de muros ascendentes se midieron en nueve ubicaciones en los pisos 5 y 16. Las mediciones se realizaron durante un a�o en cuatro per�odos de cuatro semanas cada uno. Los resultados demostraron que las concentraciones atmosf�ricas de especies qu�micas no variaban con la altura. El n�meros de concentraciones atmosf�ricas de > 0,5μm part�culas corroboraron este resultado. Aunque no se reflej� en la informaci�n qu�mica, los n�meros de concentraci�n de part�culas de > 5μm medidos en el piso 16 eran en promedio un 30% mayores que aquellas en el piso 5. Las velocidades espaciales de deposici�n de SO2 mayores y menores, nunca difirieron en m�s de un factor de dos en los diferentes per�odos de tiempo. Las diferencias relativas en velocidades de sedimentaci�n de una ubicaci�n a otra fueron consistentes en todos los experimentos del muestreo. En el piso 16 las velocidades de sedimentaci�n fueron mayores que aquellas del piso 5 debido, al menos en parte, al hecho de que las ubicaciones del piso 16 estaban m�s expuestas al viento. La ausencia de gradientes sugiere que los patrones de adquisici�n de suciedad del edificio est�n determinados por los procesos de sedimentaci�n de contaminantes y el lavado de la lluvia, compitiendo entre si. Esta hip�tesis se sustenta comparando los patrones de adquisici�n de suciedad del edificio en los a�os 30 con patrones recientes. Fotograf�as de archivo muestran mayor cantidad de suciedad, consistente con los mayores niveles de poluci�n atmosf�rica que exist�an entonces. Los resultados de este estudio pueden contribuir al dise�o de protocolos de limpieza y tratamientos para los edificios con geometr�as similares, en medio-ambientes similares. 1 INTRODUCTIONSeveral types of building stone deterioration have been well documented, including discoloration, erosion of material, and changes in the physical and chemical characteristics of the surface. Developing strategies to prevent this deterioration requires knowledge of the processes by which the damage occurs—for example, by deposition of air pollutants or by biological growth on the stone surface. Furthermore, the choice of cleaning and restoration techniques depends on the processes causing the damage. Differentiating between pollutant deposition and biological growth is difficult and generally requires on-site testing. Unfortunately, getting access to the building walls sometimes demands scaffolding, and due to expense scaffolds are typically not erected until shortly before restoration work begins. Thus, early identification of the primary deteriorating or discoloring agents is often difficult and tentative. In this study, field measurements of air pollutant concentrations and deposition are used in conjunction with archival photographs to draw conclusions regarding the role of pollutants in the soiling of a tall building. The structure of interest is the 42-story Cathedral of Learning, a National Historic Landmark on the University of Pittsburgh campus (fig. 1). The building is made of Indiana limestone and was constructed between 1929 and 1937. Since the time of construction, there have been numerous air pollution sources within a few kilometers of the building. These include steel manufacturing plants that employ coke ovens and blast furnaces, a coal-burning steam plant, heavy motor vehicle traffic, coal-burning railroads and riverboats, and a large number of domestic coal combustion sources such as home furnaces.
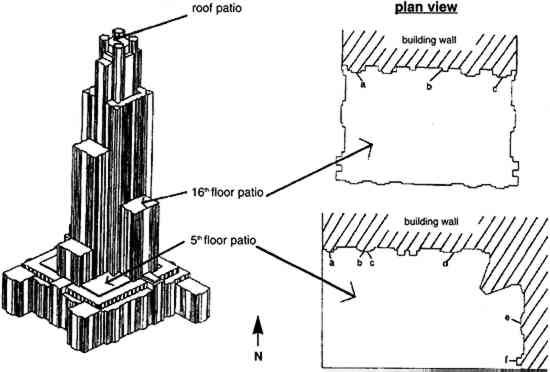
At present, two sides of the Cathedral of Learning have extensive soiling, particularly on the lower two-thirds of the building. In a study on the alteration crust at the cathedral, McGee (1995, 1997) and Etyemezian et al. (1998b) report that iron-, silicon-, and aluminum-rich fly ash particles are found in samples of soiled surfaces and that such particles are much less prevalent in samples of unsoiled surfaces. This result indicates that surface soiling at the cathedral is primarily due to the deposit of anthropogenic particles to the building walls. This research had three major objectives. First, we wanted to identify the extent to which airborne concentrations of certain pollutants vary with height on the cathedral. The pollutants of interest include SO42− particles, carbon particles, sulfur dioxide gas (SO2), total NO3− (HNO3 gas and NO3− particles), and total particle number. Such information can provide insight into the relative importance of local and regional sources of pollutants as well as pathways for delivery of pollutants to the building surface. Second, we wished to examine variations in dry deposition of SO2 with height and location. This information can provide insight into whether the variability in pollutant deposition is partly responsible for the observed soiling patterns. Third, we wished to consider long-term variations in soiling patterns on the building in light of changes in pollution concentrations. This part of the project made use of previously obtained historical pollutant data as well as archival photographs. Such information enabled us to investigate the roles of pollutant deposition and subsequent wash-off by rain in affecting the soiling. Although this study focused on only one building, the results may also be applicable to similarly structured buildings in similar environments. 2 BACKGROUNDCalcareous stones exposed to the atmosphere are vulnerable to attack by several processes that occur naturally. These processes include microbial activity on the stone surface, dissolution by rain, and physical stresses such as freezethaw cycles. Anthropogenic air pollutants are frequently responsible for accelerated deterioration, both directly through physical and chemical attack, and indirectly by providing substrates for microbial growth. In recent years, considerable attention has been given to the role of biological agents in damage to buildings (e.g., Wilmzig and Bock 1995; Freemantle 1996; Mitchell et al. 1996; Young 1996b). In general, species of fungi, algae, lichens, and bacteria have been found on surfaces of building stones (Bock and Sand 1993). These organisms can accelerate deterioration either by physical processes such as alteration of the normal wetting-drying cycle (Young 1996a) or by chemical processes such as mineral and organic acid production and the secretion of metal-chelating agents (Palmer et al. 1991). It is difficult to estimate the quantities and overall effects of biodeteriogens, in part because the fecundity and productivity of these organisms are strongly dependent on microenvironmental factors. These include insolation, stone type and porosity, surface and air temperatures, availability of a suitable substrate, and availability of water from incident rain, stone pore capillarity, or condensation and evaporation cycles (Bock and Sand 1993). In addition to the expected temporal variability caused by changes in the weather (Tayler and May 1991), there can be considerable spatial variability over short distance scales. Understanding biodeterioration processes is further confounded by a possible correlation between air pollution levels and biodeterioration rates (Young 1996a). For example, Warscheid et al. (1991) have shown that some chemo-organotrophic bacteria isolated from sandstones of historic monuments are able to utilize petroleum derivatives as sources of carbon as well as energy. Several categories of air pollutants can accelerate the natural deterioration of stone through two primary processes: wet deposition and dry deposition (e.g., Amoroso and Fassina 1983; Sherwood et al. 1990). The former refers to the deposition of a pollutant by a precipitation process such as rain or snow; acid rain is an example. Several authors have considered the effects of acid rain on calcareous stones (Braun and Wilson 1970; Livingston 1992; Hutchinson et al. 1993; Mossotti and Eldeeb 1994; Winkler 1996). Dry deposition includes those processes by which pollutants are transported to the surface in the absence of precipitation and become physically or chemically bound to the surface. Damage to calcareous building stone by dry deposition has been attributed largely to sulfur dioxide gas (SO2). For example, Meierding (1993) found that mean surface recession rates of century-old Vermont marble tombstones in the United States were well correlated with SO2 concentrations. In addition, some authors point out that nitric acid gas (HNO3) may also be sorbed onto a carbonate surface (Fenter et al. 1995; Kirkitsos and Sikiotis 1995). The removal of SO2 by certain stone types is a well-documented phenomenon (Judeikis et al. 1978). Calcareous stones subjected to high relative humidity develop a moist surface layer where SO2 can readily dissolve (Spedding 1969; Spiker et al. 1995); in general, the rate of dissolution increases at higher relative humidities and wind speeds (Spiker et al. 1995). Dissolved SO2 can then oxidize to form a sulfite (SO32−) or sulfate (SO42−) species. The oxidation process results in the production of acid, which can cause the calcium carbonate (CaCO3) in the stone to dissolve. When calcium ions (Ca2+) combine with SO32− or SO42−, CO32− is effectively displaced from the stone surface. This process, known as sulfation, may also involve gaseous and particulate air pollutants other than sulfur species. Gases such as ozone (O3) (Haneef et al. 1992) and nitrogen dioxide (NO2) (Johansson et al. 1988) have been shown to increase SO2 deposition to limestone. Surface crust analyses of damaged stone have also shown a close relation between deposited anthropogenic particles and the formation of gypsum crystals (Del Monte et al. 1981; Zappia et al. 1993; Sabbioni 1994), suggesting a relationship between sulfation and the presence of airborne particles. However, Hutchinson et al. (1992) have reported that limestone seeded with coal fly ash or transition metal oxide catalysts is not susceptible to elevated SO2 deposition. These authors suggest that seeding stone samples with oxidation catalysts has a negligible effect because natural stones already contain high levels of impurities. In contrast, seeding pure CaCO3 with metal oxide catalysts does increase the rate of sulfation. Urban air pollution studies have considered effects of buildings on dispersion of vehicle emissions as well as dispersion of individual plumes from stationary sources. In general, dispersion of vehicle emissions in street canyons is a function of the building height divided by the street width, known as the aspect ratio (Lee and Park 1994), as well as the geometric configurations of city blocks, the ambient wind direction, and the movement of motor vehicles (DePaul and Sheih 1986; Dabberdt and Hoydysh 1991; Hoydysh and Dabberdt 1994). Qin and Chan (1993) and Qin and Kot (1993) have reported that significant differences in carbon monoxide and nitrogen oxides (NOx) concentrations exist between the top and bottom of buildings surrounding street canyons in Guangzhou, China. Qin and Kot (1990) have also shown that vehicle traffic near a 31-story (100 m) tower can result in elevated NOx concentrations near the downwind building surface up to a height of 66 m. The effect of a building on the dispersion of a stationary source plume is, in general, dependent on the building geometry, source location, and prevailing wind conditions (e.g., Huber et al. 1991; Lee et al. 1991; Thompson 1993). In some cases, direct measurement of the spatial variability of air pollutant concentrations may be easier than application of theoretical considerations. 3 EXPERIMENTALVertical gradients of pollutant concentrations and deposition fluxes were measured by sampling at varying elevations on the Cathedral of Learning. Three locations were chosen for sampling: the 5th floor and 16th floor patios on the southeastern facade and the patio on the roof (fig. 2). The southeastern facade was chosen for two reasons. First, it was one of the two heavily soiled sides of the building (the northeastern facade being the other). Second, the 5th floor and 16th floor patios on the southeastern facade were frequently on the windward side of the cathedral (fig. 3) where mixing is not affected by the wake cavity of the building.
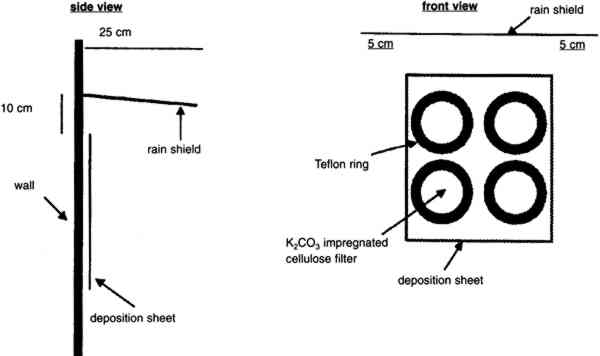
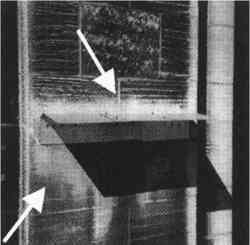
The vertical gradient experiments were conducted on four separate occasions between November 20, 1995, and August 21, 1996. The experiments were scheduled so that representative sets of data were obtained during the different seasons of the year (table 1). Each set of experiments included four consecutive sampling periods of 1 week each. Sampling was uninterrupted throughout this period except for approximately 3 hours each week for changing samples. Airborne concentrations of SO42− particles, SO2 gas, and total NO3− species (NO3− particles and HNO3 gas) were measured. Airborne concentrations of elemental and organic carbon particles were measured during the fall and winter experiments only. For the spring and summer experiments, polycarbonate membrane filters were used in place of the carbon measurements to obtain samples for scanning electron microscopy (SEM) analysis. Laser particle counters were used to provide real-time data for number concentrations of particles with diameter > 0.5 μm and > 5 μm. Each set of experiments also included two 2-week measurements of SO2 deposition fluxes. TABLE 1. SAMPLING SCHEDULE AT THE CATHEDRAL OF LEARNING All sampling was conducted using identical sets of sampling equipment at each site, two replicate sets for airborne concentrations and four sets for deposition fluxes. Airborne concentrations were measured on patios on the 5th floor, 16th floor, and roof of the cathedral. Deposition flux and laser particle counter measurements were conducted on the 5th and 16th floors only. 3.1 AIRBORNE CONCENTRATIONS OF CHEMICAL SPECIESMany of the procedures for measuring concentrations at the cathedral were developed by Gould et al. (1993). Air samplers were placed on supports 1.5 m above the surface of the patios. Concentrations of anions were measured using multistage Teflon filterpacks (Savillex Corp.) with 47 mm diameter filters. Each filterpack included a Teflon Zefluor filter (Gelman P5PL047, 1 μm pore size) followed by two back-to-back nylon Nylasorb filters (Gelman 66509). These were followed by two sets of potassium carbonate impregnated cellulose filters (Whatman 1441-047, ashless), with each set consisting of two back-to-back filters. The Teflon filters were used to sample SO42− and NO3− particles, while the nylon and cellulose filters were used to sample HNO3 and SO2, respectively. A critical flow orifice maintained the flow at 1 liter per minute. Stainless steel filter holders (Millipore XX50-047-10, open-faced) were used with 47 mm diameter quartz fiber filters (Pallflex 2500 QAT-UP) to measure carbon. A critical flow orifice maintained the flow at 3 liters per minute. These same filter holders were used with polycarbonate membrane filters (Costar Nuclepore PC-MB-47 mm, 0.4 μm pore size) for SEM analysis. A metering valve (Hoke 1656 G4YA) was used to maintain the flow at 0.2 liters per minute through the membrane filters. For all filter sampling systems, a dry test meter (Singer DTM-115) was used to obtain accurate flow rates at the beginning and end of each sampling run. The Teflon, nylon, quartz, and polycarbonate filters were used as received from the manufacturer without washing. The cellulose filters were immersed in a solution consisting of 76 ml deionized (DI) water, 24 ml glycerin, and 15 g K2CO3. The filters were then dried on a hot plate covered with clean aluminum foil. Dry filters were placed in clean polyethylene bags (Clean Room Products 6 MIL-0406), heat-sealed, and stored in a dessicator. One batch of cellulose filters was prepared at the beginning of each of the four sets of sampling experiments and used throughout that set. Filterpacks and associated tubing were washed using three rinses with DI water, one rinse with methanol (Fisher Scientific, Optima grade), 30-minute sonication in a DI water bath, and two subsequent rinses with DI water. All procedures after washing such as drying, loading, assembling, and unloading of the filterpacks were performed inside a laminar flow hood. Filters were handled only with clean Teflon-coated tweezers. Assembled filterpacks were placed in clean polyethylene bags. Field blanks of all filters were prepared in the same manner as the samples. Air was drawn through the blanks for 3-8 minutes prior to each sampling period. When unloading the filterpacks, the same contamination control procedures were observed. The Teflon, nylon, and cellulose filters were placed in clean 30 ml polypropylene bottles. The two sets of back-to-back cellulose filters were placed in separate bottles, and each set was analyzed individually. The quartz fiber and polycarbonate membrane filters were unloaded into clean 47 mm polypropylene petri dishes. With the exception of the polycarbonate membrane samples, all filters were frozen for later analysis. The quartz fiber filters were shipped frozen to the Desert Research Institute for analysis by the thermal optical reflectance method (Chow et al. 1993). The Teflon, nylon, and cellulose filters were analyzed by ion chromatography. Extractions were performed in a laminar flow hood. The upstream faces of the Teflon filters were wetted with 1 ml methanol, and 29 ml of DI water were subsequently added to the sample bottle. Thirty ml of 0.003 M sodium hydroxide and 30 ml of 0.05% hydrogen peroxide were added to the bottles containing the nylon and cellulose filters, respectively. Sample bottles were sonicated for 45 minutes. After sonication, the nylon and Teflon filters were removed from the sample bottles and discarded. The cellulose filters remained immersed in the extract solution. Extract solutions were refrigerated in order to maintain sample integrity. Ion chromatography analysis was generally performed within 2 or 3 days of the extractions. Analyses were performed on a Dionex 4500i ion chromatograph using a 4 mm AS4A anion column. Samples and standard solutions for calibrating the instrument were manually injected. After each injection, the syringe was rinsed with DI water several times. A standard solution was analyzed at least once every 2 hours. All sample handling occurred in a laminar flow hood. 3.2 LASER PARTICLE COUNTERSTwo TSI model 3755 laser particle counters (LPC) were used in these experiments. The LPCs were mounted with the nozzle side facing down at a height of 1.5 m above the patios. A laptop computer was used to log particle count data on a 2-minute basis. Some data were not properly logged during sampling periods either because the laptop did not record LPC signals or because the inlet was clogged with large particles. Problematic data were easily recognized and discarded. High particle concentrations measured by the LPC had to be corrected for double counts in accordance with the manufacturer's recommendations. 3.3 VERTICAL DEPOSITION SAMPLINGDeposition fluxes of SO2 were measured on 125 mm potassium carbonate impregnated cellulose filters (Whatman 1441-125, ashless). Four of these filters were mounted on a thin Teflon-coated aluminum plate. Flat Teflon rings (inner diameter = 105 mm) were placed on top of the filters. Labeling tape was used to fasten the rings to the plate. The fully assembled plates were transported to the cathedral in a polyethylene tray encased in a clean bag. Field blanks were exposed for approximately 10 minutes in the same manner as the samples. As with the airborne concentration filters, loading, unloading, and drying of equipment were performed on a clean surface in a laminar flow hood. Extraction and ion chromatography analyses were conducted in the same manner as for the airborne concentration cellulose filters, except that 120 ml of hydrogen peroxide extraction solution was used for each filter. Nine Teflon-coated aluminum plates were exposed simultaneously in each deposition sampling period. The samples were placed at six locations on the 5th floor (5a–5f) and three locations on the 16th floor (16a-16c) (see fig. 2). Locations 5a–5d and 16b were in areas where visible soiling was present, while the remaining four were in areas that were free of soiling. To avoid exposure to rain, each Teflon-coated aluminum plate was placed under a galvanized aluminum rain shield (fig. 4). Locations 5b and 5c were adjacent and were placed under one larger rain shield. Previous work has shown that rain shields used in different configurations may affect SO42− particle dry deposition (Davidson et al. 1985). The effect of the rain shields on SO2 gas deposition was considered by comparing fluxes to the top two and bottom two deposition surfaces for all sampling locations. If the rain shield had interfered with SO2 delivery, then deposition to the upper surfaces should have been different from deposition to the lower surfaces. The average fluxes to the top and bottom surfaces were not found to differ with statistical significance.
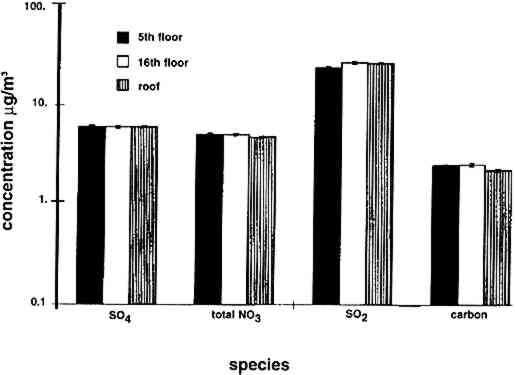
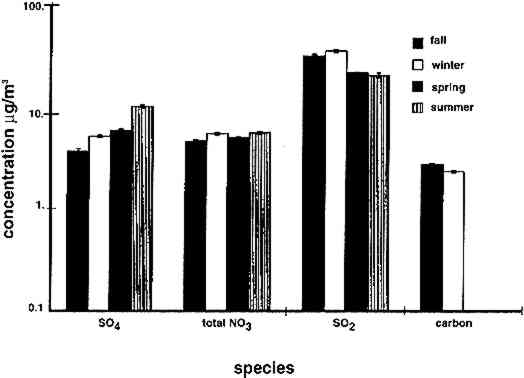
4 RESULTS4.1 AIRBORNE CONCENTRATIONS OF CHEMICAL SPECIESAirborne concentrations of SO42− particles, elemental carbon particles, SO2 gas, and total NO3− are shown in figures 5 and 6. Results of SEM analyses of the polycarbonate membrane filters are presented in a separate paper (Etyemezian et al. 1998b). Averages and standard deviations of concentrations are based on the two side-by-side replicate samplers. When one of the replicate samplers malfunctioned, the concentration was obtained from a single sample. The standard deviation for a single sample is approximated by the concentration multiplied by the average coefficient of variation (COV) for all samples for which replicates are available (44 of 48 samples). The COV has been calculated as the standard deviation divided by the average concentration. Each sample has been blank-corrected by subtracting the average mass of analyte found on field blanks from the mass of analyte found on the sample (table 2).
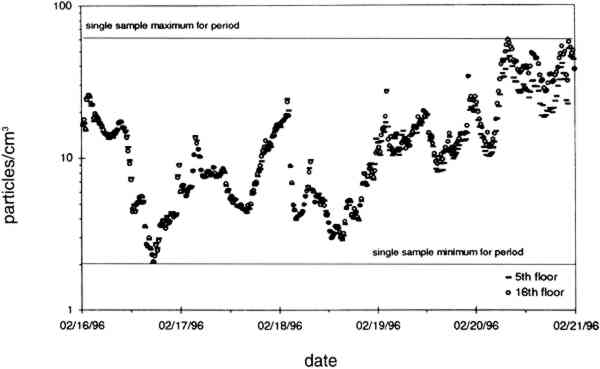
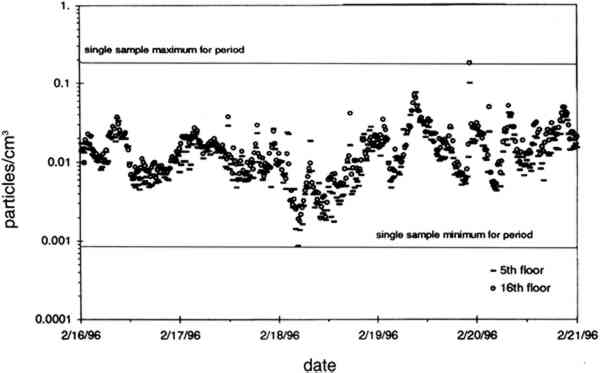
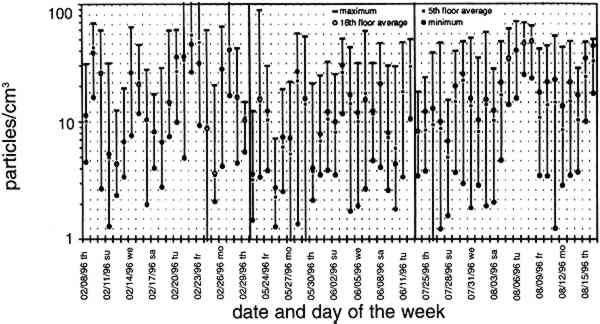
TABLE 2. ANALYTICAL DETECTION LIMITS, AVERAGE SAMPLE MASS, AND AVERAGE FIELD BLANK MASS Several authors have documented artifacts associated with NO3 species measurement using staged filterpack systems (Appel et al. 1981; Mulawa and Cadle 1985; Hering et al. 1988). For example, volatile NO3− aerosol deposited on Teflon filters may subsequently evaporate. The vapor then redeposits on the downstream nylon filters, resulting in overestimated HNO3 gas and underestimated NO3− particle concentrations. In order to account for possible sampling artifacts, NO3 species concentrations from the experiments reported here are conservatively expressed as total NO3− (HNO3 gas and NO3− particles) by summing values from the Teflon and nylon filters. SO2 concentrations are based on the chemical analyses of both the nylon and cellulose filters, since the nylon filters tend to remove some SO2 from the airstream (Chan et al. 1986; Cadle and Mulawa 1987). Experiments in the fall of 1995 showed that two sets of cellulose filters may be needed to capture all of the SO2 at high concentrations. Therefore, a second pair of cellulose filters was added downstream of the first set for the latter part of the fall experiments and all remaining runs. As with the other airborne concentration data presented here, the standard deviations of elemental carbon concentrations reflect the variability between two side-by-side replicate airborne concentration samplers. However, only 10 of the 48 samples and 5 of the 19 field blanks have had replicate chemical analyses. Therefore, the standard deviations of carbon mass on each filter are approximated by the average COV of the samples for which replicate chemical analyses have been performed. 4.2 LASER PARTICLE COUNTSExamples of particle counts for the period February 16–20, 1996, appear in figure 7. Although particle concentration data are available on a 2-minute average basis, the concentrations in figure 7 have been averaged over 10 minutes to improve legibility. Daily average, maximum, and minimum particle concentrations are plotted in figure 8, and weekday vs. weekend particle concentrations are presented in table 3.
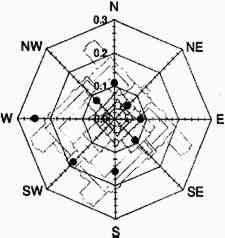
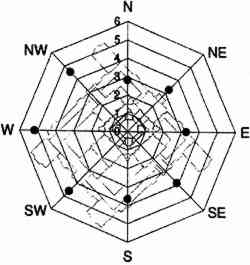
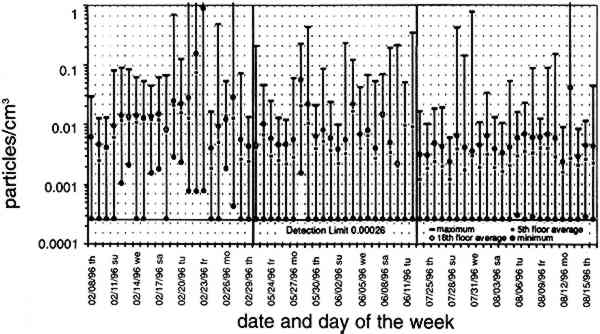
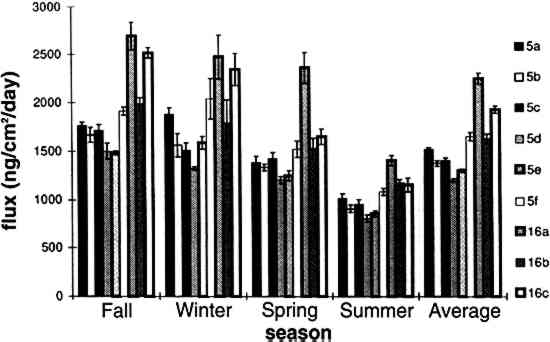
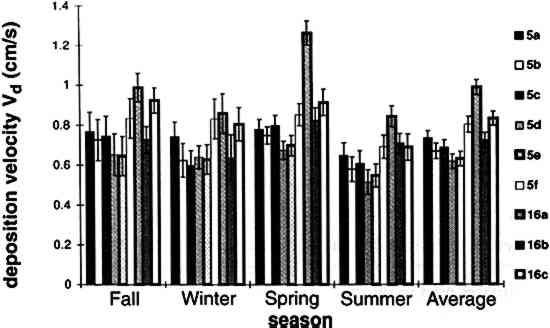
TABLE 3. WEEKDAY, SATURDAY, AND SUNDAY AVERAGE PARTICLE CONCENTRATIONS 4.3 VERTICAL DEPOSITION FLUX AND VERTICAL DEPOSITION VELOCITYMeasured vertical deposition fluxes and deposition velocities appear in figures 9 and 10. SO2 flux averages and standard deviations are based on the four replicate cellulose filters on each Teflon-coated aluminum plate. Vertical deposition flux is a measure of how much SO2 has deposited onto the surrogate surface per unit area per unit time. The deposition velocity Vd is calculated by dividing the deposition flux by the airborne concentration. The average deposition velocity and standard deviation have been calculated using:
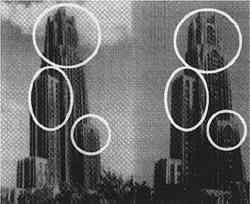
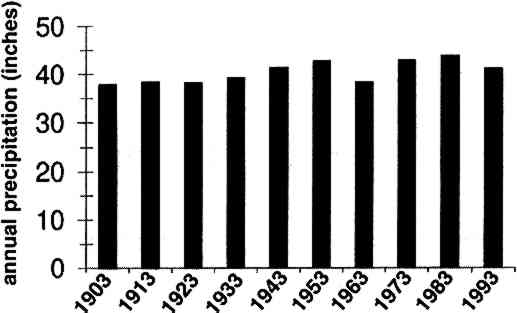
The average airborne concentration C used for calculating Vd on either the 5th or 16th floor is based on the two 1-week airborne concentrations measured on the corresponding floor. It is assumed that this average airborne concentration applies to all of the flux measurement sites on that patio. This is a reasonable assumption based on the agreement between the two replicate sets of filters sampled on each patio. The surrogate surfaces used in this study are considered to be perfect sinks for SO2, and thus SO2 is assumed to be instantaneously and completely removed when it reaches the filter. The deposition velocity is thus only a measure of gas phase mass transport from the atmosphere to the surrogate surfaces and does not include any possible surface resistance. Since limestone is not a perfect sink for SO2, the deposition velocities to the stone surface will be lower than those measured using surrogate surfaces. 5 DISCUSSION5.1 AIRBORNE CONCENTRATIONS OF CHEMICAL SPECIESThe airborne concentration data have been analyzed statistically using a two-sided t-test for averages. Significant differences in concentrations among the three sampling locations do not exist for any of the pollutants considered (table 4). TABLE 4. P-VALUES FOR TWO-SIDED T-TESTS OF SAMPLE MEAN FOR CHEMICAL SPECIES AIRBORNE CONCENTRATIONS The presence of a vertical concentration gradient requires that two conditions hold true. First, emissions from nearby sources must be sufficiently large to increase pollutant concentrations above the urban background level. Second, atmospheric mixing must be sufficiently small that vertical concentration differences can persist. SO42− and NO3− particles and HNO3 gas are generally secondary pollutants that form from chemical reactions in the atmosphere (Seinfeld and Pandis 1998). Therefore, on spatial scales corresponding to the height of the cathedral, airborne concentrations of these species are expected to be spatially homogeneous since their formation is sufficiently slow to allow mixing. SO42− and NO3− are sometimes associated with coarse particles, in part due to sorption of SO2 and HNO3 onto alkaline soil dust (Wolff 1984). The absence of a concentration gradient in SO42− particles and total NO3− suggests that if these species are associated with coarse particles, there is adequate mixing to distribute them over the height of the cathedral. The fact that elemental carbon does not exhibit a concentration gradient suggests that motor vehicle emissions from adjacent streets and the emissions from the nearby coal-fired steam plant are either rapidly mixed or else do not contribute significantly to concentrations at or above the 5th floor. To investigate the extent of pollutant mixing near the building, a limited number of measurements of vertical wind speed were conducted on the 5th and 16th floor patios on five days during the term of this project. Results show the presence of strong vertical air motions along the walls of the building on all days tested. Although preliminary, these results provide qualitative evidence of vertical mixing as a consequence of wind impinging on the cathedral. 5.2 LASER PARTICLE COUNTSIn the >0.5 μm size range, particle counts by LPC do not show a significant difference between the 5th and 16th floors (figs. 7, 8). However, >5 μm particle counts are on average 30% higher on the 16th floor than on the 5th floor. Because of their greater inertia, sampling of large particles is more sensitive to factors such as inlet angle, flow characteristics in the inlet, and ambient wind direction. Therefore, the discrepancy in >5 μm concentrations between the two floors should be regarded with caution. Note that the chemical species data do not show a difference between the 5th and 16th floors; if the >5 μm particle concentrations are indeed slightly greater on the 16th floor, then it is unlikely that there is much SO42−, NO3−, or carbon mass associated with these large particles. Figures 7 and 8 both demonstrate that particle counts can vary considerably over the course of a day. In figure 7, maxima and minima for >0.5 μm particle concentrations coincide well with those for >5 μm particles. This agreement between concentrations in the two particle sizes is found in most of the LPC data. However, changes in >0.5 μm particle concentrations are not well proportioned with those of >5 μm particles. The correlation coefficient for the data in figure 7 is 0.41, whereas the 24-hour-based correlation coefficients for all available LPC data range from −0.27 to 0.90, with a median value of 0.37. The low positive and occasional negative correlations are not surprising. Some meteorological parameters such as atmospheric stability may qualitatively affect >0.5 μm and >5 μm particle concentrations similarly. However, particles in these two size ranges are generally not emitted by the same sources and are not subjected to identical transport processes. Table 3 shows that concentrations on Saturdays and Sundays are lower than those on weekdays. This finding is consistent with the expectation of reduced motor vehicle traffic and, possibly, reduced operations of some stationary sources on weekends in the Pittsburgh area. 5.3 VERTICAL DEPOSITION FLUX AND VERTICAL DEPOSITION VELOCITIESThere is considerable spatial variability in SO2 deposition within each patio. However, most deposition velocities (Vd) are in the range 0.6–1.0 cm/s, a finding in agreement with unpublished data obtained previously at the cathedral (Lutz et al. 1994; Etyemezian et al. 1995). The values are also in agreement with measurements using the same method by Wu et al. (1992) on a bronze equestrian statue in Gettysburg National Military Park. The greatest deposition velocities on the 16th floor correspond to locations 16a and 16c. The average deposition velocities for the full year are 0.85 � 0.13 cm/s for the 16th floor, compared with 0.69 � 0.07 cm/s for the 5th floor. The sampling locations on the 16th floor are more exposed than those on the 5th floor, a difference that may, in part, be responsible for the slightly higher deposition velocities. Note that sites 5a and 5f, the most exposed sites on the 5th floor, have the highest deposition velocities on that floor. It is also of interest that the relative magnitudes of deposition velocity at one location with respect to another location do not change seasonally. For example, Vd is consistently higher at location 16c than it is at locations 5b, 5c, 5d, 5e, and 5f. Unpublished results of previous sampling at the cathedral show a similar trend for SO42− particle deposition (Gould et al. 1993; Lutz et al. 1994; Etyemezian et al. 1995). These data also show that deposition fluxes of SO42− particles to surrogate surfaces are usually at least a factor of 10 lower than those for SO2. In the earlier studies, surrogate surfaces for SO42− particle and SO2 gas collection were exposed on the 5th floor at five locations. Locations 5f, 16a, and 16c are on parts of the building where there is no visible soiling. Location 5a is inside a 0.5 m heavily soiled indent on the wall. The portion of the wall immediately outside the indent and closer to the outer corner of the patio is clean. Overall, those sampling locations with the highest deposition velocities correspond to unsoiled parts of the building surface, and these are in relatively exposed areas. Furthermore, observations of the building during rainstorms and results from a recent modeling study (Etyemezian et al. 1998a) both suggest that these exposed areas also experience the most impingement by rain. Thus, we propose that deposition of pollutants and surface rain washing are processes in dynamic competition. Archival photographs showing changes in soiling from the 1930s to the present support this hypothesis. These photographs show heavy soiling in the early years, with decreasing amounts of soiling in more recent times. Examples of two photographs, from the late 1930s and from 1995, are shown in figure 11. The first photograph shows extensive soiling on the southwestern facade. In contrast, the later photograph shows that the top one-third of the building is virtually free of soiling, and that the demarcation line between soiled and unsoiled surfaces has receded on other parts of the building. Figure 12 indicates that the amount of annual precipitation has been roughly constant over these decades. However, airborne concentrations of SO2 and particles have decreased steadily over the same time period (Davidson 1979). Thus, those areas of the facade that were soiled in the late 1930s have become unsoiled in recent years because the rate of removal of soiled material by rain washing is greater than the rate of soiling by pollutant deposition and chemical reaction. The earlier photograph suggests that the opposite was true in the late 1930s.
A study of the composition of the limestone on the Cathedral of Learning (McGee 1997) supports the hypothesis that the soiling is a direct result of pollutant deposition. McGee has collected 37 samples of black, light, and red-brown surface material for examination by optical microscopy and scanning electron microscopy with energy dispersive x-ray analysis of some samples. The black samples are composed primarily of gypsum (CaSO4) and contain numerous iron-, silicon-, and aluminum-rich fly ash particles. This finding is attributed to deposition of metal-containing particles and sulfur from pollutant sources in the area. The light surfaces are primarily calcite with only minor amounts of gypsum and metals. McGee (1997) reports that the microscopic and macroscopic appearance of light samples is similar to that of other limestone samples that are regularly washed either by rain or by routine cleaning. The color of the red-brown samples is attributed to rust from small metal spheres lodged in the stone, probably as a result of surface finishing of the limestone block. It is of interest that one of the rain shields shown in figure 4 was deployed in a soiled area on the 5th-floor patio in January 1993; since then, the surface of the stone immediately above the rain shield has become cleaned by splashing raindrops (fig. 13). Clearly, the presence of this rain shield has caused a local disturbance in the balance between pollutant deposition and surface rain washing and has enhanced the amount of washing.
5.4 APPLICABILITY OF RESULTS TO OTHER LIMESTONE BUILDINGSThe absence of a vertical pollutant concentration gradient is probably due to a combination of factors that have implications for buildings in other areas. First, it is likely that vertical mixing is enhanced by the presence of the building; tall buildings in other areas may also have strong upward air motions that can deliver pollutants generated near the ground to upper levels. Second, the location of pollution sources around the cathedral can greatly affect the airborne concentrations at the building. The cathedral is located in a major urban area with considerable local traffic as well as mobile and stationary sources for several kilometers in all directions. Thus, the contributions from nearby sources (within a few hundred meters) may be diminished by the presence of numerous regional pollution sources. Note that pollutants from several kilometers away have time to mix vertically in the atmosphere and can increase airborne concentrations at higher elevations. Effects of nearby sources may be more pronounced in cases where a building is located in a less-polluted setting. Third, the presence of nearby buildings is likely to have a major effect on dispersion near any building. The cathedral is considerably taller than the surrounding buildings and is 80 m from the nearest buildings to the north and west and over 120 m from the nearest buildings to the south and east. This isolation eliminates some of the trapping of pollutants that may occur in narrow street canyons, as reported by Lee and Park (1994). It must be noted that no measurements in the present study were conducted below the 5th floor, and thus conclusions regarding the absence of a concentration gradient apply only above this level. The concept of pollutant deposition and rain washing as competing processes has implications for other buildings. Comparing modern and archival photographs of the cathedral shows general decreases in the soiling that are qualitatively consistent with decreases in pollutant concentration. Archival photographs of buildings where soiling is due to microbial activity may show distinctly different patterns. 6 SUMMARY AND CONCLUSIONSIn order to better understand pollutant sources and transport pathways responsible for the soiling of a tall limestone building, this study has investigated whether vertical gradients in airborne pollutant concentrations and deposition fluxes currently exist at the 42-story Cathedral of Learning in Pittsburgh, Pennsylvania. The study has also considered long-term changes in soiling on the building as a means of identifying the roles of pollutant deposition and rain washing in affecting the soiling patterns. The study involved measurements of airborne concentrations of SO42− particles, elemental carbon particles, SO2 gas, and total NO3− (NO3− particles and HNO3 gas) on the 5th floor, 16th floor, and roof. In addition, SO2 deposition fluxes were measured on the 5th and 16th floors, and laser particle counters were used on these same two floors to measure airborne particle number concentrations in two size ranges: >0.5 μm and >5 μm. The experiments were conducted for 4 weeks during each of the four seasons of the year beginning in fall 1995. The airborne chemical species measurements indicate that there are no statistically significant vertical gradients for any of the pollutants. The lack of a gradient is attributed to a variety of regional and local sources that are expected to be well mixed by the time they reach the cathedral, vertical winds in the vicinity of the building, and the lack of buildings nearby that might otherwise trap pollutants and prevent vertical mixing. The laser particle counts for >0.5 μm particles likewise show lack of gradient, although the >5 μm particles show slightly greater airborne particle number concentrations on the 16th floor compared with those on the 5th floor. Deposition fluxes and deposition velocities of SO2 to surrogate surfaces show small but consistent differences among the locations sampled. Values are greatest at locations that are most exposed to the wind such as the outside corners of the patios. The values are greater on the 16th floor than on the 5th floor, partly because two of the three 16th floor sampling locations are situated on the corners of the patio. Comparison of archival with more recent photographs shows that the soiling on the cathedral has decreased over time. This observation is consistent with decreasing trends in airborne pollutant concentrations over the past several decades. It is thus likely that rain is washing soiled material off the building surface at a greater rate than chemical species are depositing and reacting with the surface. The opposite apparently was true in the 1930s, when air pollutant concentrations were considerably greater than at present. Overall, these results may be of interest to conservators who must develop strategies for cleaning and restoring building surfaces and for preventing future damage. Although the conclusions reached here are a result of testing at the Cathedral of Learning, it is likely that many of the findings also apply to buildings in other urban areas. ACKNOWLEDGEMENTSThis research was funded by the National Park Service Cooperative Agreements 042419005 and 00196035. The assistance of the Department of Facilities Management at the University of Pittsburgh and Dominic Fagnelli, the Cathedral of Learning building engineer, are greatly appreciated. The authors wish to acknowledge Susan Sherwood for her valuable suggestions and assistance over the past several years. Thanks are due to Michael Lutz and Timothy Gould for their earlier work on the Cathedral of Learning and to Mitchell Small for help with the statistical analysis. The authors also wish to thank Weiping Dai, Hampden Kuhns, Spyros Pandis, Ross Strader, and Maria Zufall for their frequent and welcome suggestions. ElizaBeth Bede and Chandra Reedy provided comments and insights that were very helpful in the writing of this manuscript. A large fraction of the airborne sampling setup was constructed by Larry Cartwright and his crew. Preshanth Mekala and Karen Pinkston contributed their time to ion chromatography analyses. The historical photograph was furnished by the Carnegie Library of Pittsburgh, Oakland Branch. Justin Parkhurst assisted with the collection of several historical photographs. The computer drawings of the cathedral that appear in figure 2 were created by Judy Lee, David Iorio, and Ivan Locke. Thomas Curry, Sean Jutahkiti, and Anthony Paul are thanked for their work on measuring vertical wind speeds near the walls of the Cathedral of Learning. REFERENCESAmoroso, G. G., and V.Fassina. 1983. Stone decay and conservation: Atmospheric pollution, cleaning, consolidation and protection, Materials Science monographs11. New York: Elsevier. Appel, B. R., Y.Tokiwa, and M.Haik. 1981. Sampling of nitrates in ambient air. Atmospheric Environment15:283–89. Bock, E., and W.Sand. 1993. A review: The microbiology of masonry deterioration. Journal of Applied Bacteriology74:503–14. Braun, R. C., and M. J. G.Wilson. 1970. The removal of atmospheric sulphur by building stones. Atmospheric Environment4:371–78. Cadle, S. H., and P. A.Mulawa. 1987. The retention of SO2 by nylon filters. Atmospheric Environment21:599–603. Chan, W. H., D. B.Orr, and D. H. S.Chung. 1986. An evaluation of artifact SO42− formation on nylon filters under field conditions. Atmospheric Environment20:2397–2401. Chow, J. C., J. G.Watson, L. C.Pritchet, W. R.Pierson, C. A.Frazier, and R. G.Purcell. 1993. The DRI thermal/optical reflectance carbon analysis system: Description, evaluation, and applications in U.S. air quality studies. Atmospheric Environment27a:1185–201. Dabberdt, W. F., and W. G.Hoydysh. 1991. Street canyon dispersion: Sensitivity to block shape and entrainment. Atmospheric Environment25a:1143–53. Davidson, C. I.1979. Air pollution in Pittsburgh: A historical perspective. Journal of the Air Pollution Control Association29:1035–41. Davidson, C. I., S. E.Lindberg, J. A.Schmidt, L. G.Cartwright, and L. R.Landis. 1985. Dry deposition of sulfate onto surrogate surfaces. Journal of Geophysical Research90:2123–30. Del Monte, M., C.Sabbioni, and O.Vittori. 1981. Airborne carbon particles and marble deterioration. Atmospheric Environment15:645–52. DePaul, F. T., and C. M.Sheih. 1985. Measurements of wind velocities in a street canyon. Atmospheric Environment20:455–59. Etyemezian, V., C. I.Davidson, and S.Finger. 1995. Influence of atmospheric pollutants on soiling of a limestone building surface. Progress report for the National Park Service, Pittsburgh. September. Carnegie Mellon University, Pittsburgh, Pa. Etyemezian, V., C. I.Davidson, M. J.Zufall, W.Dai, S.Finger, and M.Striegel. 1998a. Impingement of rain drops on a tall building. Typescript. Carnegie Mellon University, Pittsburgh, Pa. Etyemezian, V., N. R.Strader, C. I.Davidson, W.Dai, S.Finger, and M.Striegel. 1998b. Deposition of particles and surface soiling at a tall limestone building. Typescript. Carnegie Mellon University, Pittsburgh, Pa. Fenter, F. F., C.Francois, and M. J.Rossi. 1995. Experimental evidence for the efficient “dry deposition” of nitric acid on calcite. Atmospheric Environment29:3365–72. Freemantle, M.1996. Historic monuments pose challenge to conservation scientists. Chemical and Engineering News74:20–23. Gould, T. R., C. I.Davidson, S.Finger, M. J.Small, and M. R.Lutz. 1993. Influence of atmospheric pollutant concentrations and deposition rates on soiling of a limestone building surface. Progress report for the National Park Service, Pittsburgh. May. Carnegie Mellon University, Pittsburgh, Pa. Haneef, S. J., J. B.Johnson, C.Dickinson, G. E.Thompson, and G. C.Wood. 1992. Effect of dry deposition of NOx and SO2 gaseous pollutants on the degradation of calcareous building stones. Atmospheric Environment26a:2963–74. Hering, S. V., D. R.Lawson, I.Allegrini, A.Febo, C.Perrino, M.Possanzini, J. E.SicklesII, K. G.Anlauf, A.Wiebe, B. R.Appel, W.John, J.Ondo, S.Wall, R. S.Braman, R.Sutton, G. R.Cass, P. A.Solomon, D. J.Eatough, E. C.Ellis, D.Grosjean, B. B.Hicks, J. D.Womack, J.Horrocks, K. T.Knapp, T. G.Ellestad, R. J.Paur, W. J.Mitchell, M.Pleasant, E.Peake, A.MacLean, W. R.Pierson, W.Brachaczek, H. I.Schiff, G. I.Mackay, C. W.Spicer, D. H.Stedman, A. M.Winer, H. W.Biermann, and E. C.Tuazon. 1988. The nitric acid shootout: Field comparison of measurement methods. Atmospheric Environment22:1519–39. Hoydysh, W. G., and W. F.Dabberdt. 1994. Concentration fields at urban intersections: Fluid modeling studies. Atmospheric Environment28:1849–60. Huber, A. H., S. PalArya, S. A.Rajala, and J. W.Borek. 1991. Preliminary studies of video images of smoke dispersion in the near wake of a model building. Atmospheric Environment25a:1199–1209. Hutchinson, A. J., J. B.Johnson, G. E.Thompson, G. C.Wood, P. W.Sage, and M. J.Cooke. 1992. The role of fly-ash particulate material and oxide catalysts in stone degradation. Atmospheric Environment26a:2795–2803. Hutchinson, A. J., J. B.Johnson, G. E.Thompson, G. C.Wood, P. W.Sage, and M. J.Cooke. 1993. Stone degradation due to wet deposition of pollutants. Corrosion Science34:1881–98. Johansson, L. G., O.Lindqvist, and R. E.Mangio. 1988. Corrosion of calcareous stones in humid air containing SO2 and NO2. Durability of Building Materials5:439–49. Judeikis, H. S., T. B.Stewart, and A. G.Wren. 1978. Laboratory studies of heterogeneous reactions of SO2∗. Atmospheric Environment12:1633–41. Kirkitsos, P., and D.Sikiotis. 1995. Deterioration of Pentelic marble, Portland limestone and Baumberger sandstone in laboratory exposures to gaseous nitric acid. Atmospheric Environment29:77–86. Lee, J. T., D. L.Call, R. E.LawsonJr., W. E.Clements, and D. E.Hoard. 1991. A video image analysis system for concentration measurements and flow visualization in building wakes. Atmospheric Environment25a:1211–25. Lee, I. Y., and H. M.Park. 1994. Parametrization of the pollutant transport and dispersion in urban street canyons. Atmospheric Environment28:2343–49. Livingston, R A.1992. Graphical methods for examining the effects of acid rain and sulfur dioxide on carbonate stones. Proceedings of the 7th International Congress on Deterioration and Conservation of Stone, Lisbon, Portugal. Lisbon: LNEC. 375–86. Lutz, M. R., C. I.Davidson, S.Finger, T. R.Gould, M. J.Small, and V.Etyemezian. 1994. Influence of atmospheric pollutants on soiling of a limestone building surface, 1991–1994. Progress report for the National Park Service, Pittsburgh. September. Carnegie Mellon University, Pittsburgh, Pa. McGee, E. S.1995. Sampling protocol used at the Cathedral of Learning in Pittsburgh, Pennsylvania. USGS Open-File Report 95–672, Reston, Va. McGee, E. S.1997. Surficial alteration at the Cathedral of Learning in Pittsburgh, Pennsylvania. USGS Open-File Report 97–275, Reston, Va. Meierding, T. C.1993. Marble tombstone weathering and air pollution in North America. Annals of the Association of American Geographers83 (4):568–88. Mitchell, J. D., R.Gu, T. E.Ford, and N. S.Berke1996. Fungal degradation of concrete. Deutsche Gesellschaft f�r Chemisches Apparatewesen (DECHEMA) monographs133. Frankfurt, Germany: DECHEMA. 135–42. Mossotti, V. G., and A. R.Eldeeb. 1994. Calcareous stone dissolution by acid rain. Typescript. Menlo Park, Calif. Mulawa, P. A., and S. H.Cadle. 1985. A comparison of nitric acid and particulate nitrate measurements by the penetration and denuder difference methods. Atmospheric Environment19:1317–24. Palmer, R. J., J.Siebert, and P.Hirsch1991. Biomass and organic acids in sandstone of a weathering building: Production by bacterial and fungal isolates. Microbial Ecology21:253–66. Qin, Y., and L. Y.Chan. 1993. Traffic source emission and street level air pollution in urban areas of Guangzhou, South China (P.R.C.). Atmospheric Environment27b:275–82. Qin, Y., and S. C.Kot. 1990. Validation of computer modeling of vehicular exhaust dispersion near a tower block. Building and Environment25:125–31. Qin, Y., and S. C.Kot. 1993. Dispersion of vehicular emission in street canyons, Guangzhou City, South China (P.R.C.). Atmospheric Environment27b:283–91. Sabbioni, C.1994. Effects of air pollution on historic buildings and monuments—Scientific basis for conservation: Physical, chemical, and biological weathering. European Cultural Heritage Newsletter on Research8(1):2–6. Seinfeld, J. H., and S. N.Pandis. 1998. Atmospheric chemistry: Air pollution to global change. New York: John Wiley and Sons. Sherwood, S. I., D. F.Gatz, R. P.HoskerJr., C. I.Davidson, D. A.Dolske, B. B.Hicks, D.Langmuir, R.Linzey, F. W.Lipfert, E. S.McGee, V. G.Mossotti, R. L.Schmiermund, and E. S.Spiker. 1990. Processes of deposition to structures. Report 20 in National Acid Precipitation Assessment Program, Acidic Deposition: State of Science and Technology, vol. 3. September. Spedding, D. J.1969. Sulphur dioxide uptake by limestone. Atmospheric Environment3:683. Spiker, E. C., R. P.HoskerJr., V. C.Weintraub, and S. I.Sherwood. 1995. Laboratory study of SO2 dry deposition on limestone and marble: Effects of humidity and surface variables. Water, air, and soil pollution proceedings of the 1995 5th international conference on acidic deposition: Science and policy, acid reign '95, G�teborg, Sweden. 2679–85. Tayler, S., and E.May1991. The seasonality of heterotrophic bacteria on sandstones from ancient monuments. In Biodeterioration of cultural property, ed.R. J.Koestler. New York: Elsevier. 390–91. Thompson, R. S.1993. Building amplification factors for sources near buildings: A wind tunnel study. Atmospheric Environment27a:2313–25. Warscheid, T., M.Oelting, and W. E.Krumbein. 1991. Physico-chemical aspects of biodeterioration processes on rocks with special regard to organic pollutants. In Biodeterioration of cultural property, ed.R. J.Koestler. New York: Elsevier. 397–99. Wilmzig, M., and E.Bock. 1995. Endangerment of mortars by nitrifiers, heterotrophic bacteria and fungi. In Biodeterioration and biodegradation 9, ed.A.Bousher et al. Rugby, UK: Institution of Chemical Engineers. 195–97. Winkler, E. M.1996. Important agents of weathering for building and monumental stone. Engineering Geology1:381–400. Wolff, G. T.1984. On the nature of nitrate in coarse continental aerosols. Atmospheric Environment18:977–81. Wu, Y. L., C. I.Davidson, D. A.Dolske, and S. I.Sherwood. 1992. Dry deposition of atmospheric contaminants: The relative importance of aerodynamic, boundary layer, and surface resistances. Aerosol Science and Technology16:65–81. Young, P.1996a. Mouldering monuments. New Scientist152:37–38. Young, P.1996b. Pollution-fueled “biodeterioration” threatens historic stone. Environmental Science and Technology30:206a–8a. Zappia, G., C.Sabbioni, and G.Gobbi. 1993. Non-carbonate carbon content on black and white areas of damaged stone monuments. Atmospheric Environment27a:1117–21. SOURCES OF MATERIALSPolyethylene BagsClean Room Products Inc., 1800 Ocean Ave., Ronkonkoma, N.Y. 11779-6532 Polycarbonate Membrane FiltersCostar, One Alewife Center, Cambridge, Mass. 02140 Ion ChromotagraphDionex Corp., 228 Titan Way, Sunnyvale, Calif. 94088-3603 MethanolFisher Scientific, 711 Forbes Ave., Pittsburgh, Pa. 15219-4785 Teflon Zefluor and Nylasorb FiltersGelman Sciences, 600 S. Wagner Rd., Ann Arbor, Mich. 48106 Metering ValveHoke, One Tenakill Park, Cresskill, N.J. 07626 Stainless Steel Filter HoldersMillipore Corp., 80 Ashby Rd., Bedford, Mass. 01730 Quartz Fiber FiltersPallflex Corp., 125 Kennedy Dr., Putnam, Conn. 06260 Teflon Filter PacksSavillex Corp., 6133 Baker Rd., Minnetonka, Minn.55345 Dry Test MeterSinger Corp., Product division closed Laser Particle CountersTSI Inc., P.O. Box 64394, St. Paul, Minn. 55164 Potassium Carbonate Impregnated Cellulose FilterWhatman, 9 Bridgewell Place, Clifton, N.J. 07014 AUTHOR INFORMATIONVICKEN ETYEMEZIAN is a doctoral student at Carnegie Mellon University (CMU). He received combined bachelor degrees in physics and engineering from Occidental College and the California Institute of Technology, respectively, and a master's of science in environmental engineering from the Johns Hopkins University. His research has focused on the effects of atmospheric pollutants on sensitive building materials, with an emphasis on dry deposition and surface rain washing. Some of his other interests include policies for sustainable environments in developing countries, urban and remote atmospheric chemistry, contaminant transport in groundwater, and innovative solutions for wastewater treatment. Address: Department of Civil and Environmental Engineering, Carnegie Mellon University, Pittsburgh, Pa. 15213. CLIFF I. DAVIDSON is professor of civil and environmental engineering and engineering and public policy at Carnegie Mellon University (CMU). He is also director of the Environmental Institute at CMU. His main research interests involve atmospheric pollutants, focusing on the way particles and gases are transported through the atmosphere, the mechanisms by which particles and gases deposit from the atmosphere onto different types of surfaces, and historical trends in air pollution levels. His main educational activities include developing material that can be used broadly in basic science and engineering courses to increase student awareness of environmental issues. Address as for Etyemezian. SUSAN FINGER is on the faculty of the Civil and Environmental Engineering Department at Carnegie Mellon University. She is also affiliated with the Engineering Design Research Center, the Robotics Institute, and the Department of Mechanical Engineering. She is a founder and co-editor-in-chief of the journal Research in Engineering Design. Her research interests include representation languages for designs, integration of design and manufacturing concerns, and computer-aided engineering. Address as for Etyemezian. MARY F. STRIEGEL currently serves as the materials research program coordinator for the National Center for Preservation Technology and Training, where she is responsible for the supervision of in-house and external research on the effects of air pollution on cultural resources. Before going to NCPTT, she was a researcher with the Getty Conservation Institute. She holds bachelor degrees in fine arts and chemistry from the University of Louisville, a master's degree in analytical chemistry from Indiana University–Purdue University in Indianapolis, and a doctoral degree in inorganic chemistry from Washington University in St. Louis. Address: National Center for Preservation Technology and Training, NSU, Box 5682, Natchitoches, La. 71497. NOEMI BARABAS received a bachelor's of science degree in chemical engineering and public policy with a minor in environmental engineering from Carnegie Mellon University. She is completing work on a master's degree (with subsequent Ph.D.) in environmental engineering in the Water Resources and Environmental Engineering Program at the University of Michigan. Her interests are water resource management and policy and its application in Eastern Europe. Address: Department of Civil and Environmental Engineering, University of Michigan, 116 EWRE Building, 1351 Beal Ave., Ann Arbor, Mich. 48109. JUDITH C. CHOW, research professor in the Energy and Environmental Engineering Center (EEEC) at the Desert Research Institute (DRI), University and Community College system of Nevada, has more than 19 years of experience in conducting air quality studies and performing statistical data analysis. She directs DRI's Environmental Analysis Facility, where she supervises filter processing and chemical operations. Dr. Chow has extensive experience in program planning; ambient and source sampling from urban and nonurban areas; gravimetric, x-ray fluorescence, atomic absorption spectrophotometric, ion chromatographic, automated colorimetric, and thermal/optical reflectance carbon analyses of filter samples, as well as interpretive statistical data analyses, principal component analyses, such as multivariate statistical analysis, and chemical mass balance receptor modeling. Address: Desert Research Institute, 5625 Fox Ave., P.O. Box 60220, Reno, Nev. 89506.
 Section Index Section Index |