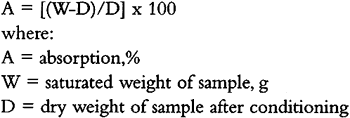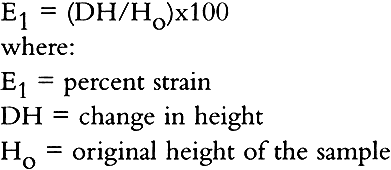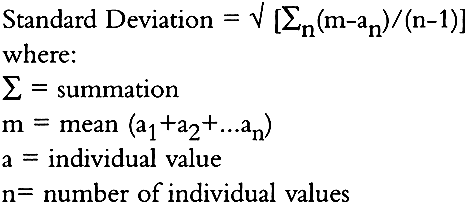FILLS FOR WHITE MARBLE: PROPERTIES OF SEVEN FILLERS AND TWO THERMOSETTING RESINSELEONORA E. NAGY
APPENDIX1 APPENDIX: ANALYTICAL PROCEDURES1.1.1 DensityThe dimensions of the samples were measured with a metric dial caliper, 0.05 mm–130 mm, Type 6910. The samples were weighed on a Model 333 Acculab electronic digital scale with a minimum sensitivity of 0.1g. The density of the samples was calculated in grams per cubic centimeter (g/cm3). 1.1.2 Water SensitivityAll dry, conditioned samples and controls were weighed (as described in section 1.1), then immersed in a distilled-water bath using one plastic screen on the bottom and one on the top of each sample. The screen was necessary to weigh down the extremely lightweight samples of Globe-o-sil, Microspheres, and Eccospheres (these floated on top of the water) and allow sufficient absorption of water from the bottom of the dish. After 48 hours of immersion, the samples were removed from the bath, dried by blotting with a dry cloth, and then immediately weighed to the nearest 0.0001 g. For this purpose a Mettler H20T balance with a weight indication of 0.01 mg to 160 g was used. The specimens were weighed in the same order every time, at intervals of 6, 12, 19, 26, 33, 48, and 72 hours after immersion. The samples were left on a plastic lattice to air dry. A sample was considered dry when three successive measurements yielded the same reading. The first of these readings is indicated as dry state. The percentage absorption was calculated using a standard absorption formula for water (ASTM 1995a).
1.1.3 ColorThe colorimetry of the visible spectrum was carried out on a Macbeth Series 1500 Spectrophotometer using the CIE L∗a∗b∗ 1976 color system (ASTM 1995b). CIELAB 1976 color space is an analytical color measurement system for comparing or reproducing colors. In this system the scales on the three orthogonal axes (L∗ lightness, a∗ green-red, b∗ blue-yellow) are relatively uniform in comparison to other chromatic systems. Pulsed-xenon CIE65 was used as illumination with average daylight 6500�K illuminants, which included UV. A white standard (No. 051025) was used for calibration. The middle area of the largest surface was tested on each sample. The instrument displayed all L∗,a∗,b∗, and DE∗ values. Wet colorimetry samples were immersed as described in section 1.1. Readings were obtained using the spectrometer as described above. 1.1.4 Stress-Strain Behavior (Elasticity and Plasticity)Most of the samples were tested using a Unite-O-Matic compressive testing machine with a load of 0.00002–10,000 lbs. For samples of 1.0% concentration, a Wykeham Farrance Compressive machine equipped with a millivolt reader was used. Compressive strength and strain tests were conducted at the Engineering Department at Queen's University, Kingston, Canada. The samples “stood” in the machine in order to have the long dimension of the samples parallel to the axis of the compressive force. This meant that in the case of the natural stone samples the compressive force was parallel to the bedding. Compressive strengths were calculated using the standard formulas (ASTM 1995c).
The maximum speed of the loading head was 0.05 in./min. for the first and 0.045 in./min. for the second machine. Readings were taken at every 5 or 10/1000 of an inch load movement, depending on the strength of the sample. The percent strain was calculated as follows:
2 STANDARD DEVIATIONStandard deviation values were calculated as follows:
REFERENCESAkemi Plastics, Inc.1988. Material safety data sheet and physical and mechanical properties of the polyester-based products, no. 45-R0338. January 31. ASTM. 1995a. Standard test method for absorption of chemical-resistant mortars, grouts and monolithic surfacing, C 413-94. In Annual book of ASTM standards, vol. 6.05. Philadelphia: American Society for Testing and Materials. ASTM. 1995b. Standard test method for calculation of color differences from instrumentally measured color coordinates, D 2244-93. In Annual book of ASTM standards, vol. 6.01. Philadelphia: American Society for Testing and Materials.
Badham, M.1991. X-ray identification of marble. X-ray identification report of the calcite marble specimen. Kingston, Ontario: Miller Museum of Geology, Queen's University. Barclay, R., and C.Mathias. 1989. An epoxy/microballoon mixture for gap filling in wooden objects. Journal of the American Institute for Conservation28: 31–42. Domaslowski, W.1988. Thermosetting resins as used in stone conservation. In The deterioration and conservation of stone: Notes from the international Venetian course on stone restoration, ed.L.Lazzarini and R.Pieper. Rome: UNESCO. 329–49. Domaslowski, W.1995. Technology of epoxy mortars at low temperature and high relative humidity. Zabytkoznawstwo I konserwatorstwo15 (189): 33–63. Down, J. L.1986. The yellowing of epoxy resin adhesives: Report on high-intensity light aging. Studies in Conservation31:159–70. Larson, J.1979. The conservation of alabaster monuments in churches. Conservator2 (28):20–25. Naud�, V.1991. Report on the discussion about stone. AIC Objects Specialty Group postprints. American Institute for Conservation Annual Meeting, Albuquerque, N. M., June 8. Washington, D.C.: AIC. 1:111–12. Technology Organization, Inc.1995. Natural stone roundup: Guide to sources and fabrications of natural stones and to suppliers of products for their care/conservation. Technology and Conservation12 (3): 20–22. Tennent, N. H,.and J. H.Townsend. 1984. The significance of the refractive index of adhesives for glass repair. In Adhesives and consolidants, ed.N.Bromelle, E. M.Pye, P.Smith, and G.Thomson. London: IIC. 205–8. FURTHER READINGASTM. 1995c. Standard test method for compressive strength of chemical-resistant mortars, grouts, monolithic surfacing and polymer concretes, C 579-91. In Annual book of ASTM standards, vol. 4.05. Philadelphia: American Society for Testing and Materials. Brill, T. B.1980. Light: Its interaction with art and antiquities. New York: Plenum. Clifton, J. R.1984. Laboratory evaluation of stone consolidants. In Adhesives and consolidants, ed.N.Bromelle, E. M.Pye, P.Smith, and G.Thomson. London: IIC.151–55. Grattan, D. W., and R. L.Barclay. 1988. A study of gap-fillers for wooden objects. Studies in Conservation33:71–86. Nagy, E. E.1991. Effects of inorganic fillers on two thermosetting resins: Fills for deteriorated white marble. Unpublished typescript. Queen's University, Kingston, Ontario. Remillard, F.1984. The effect of solvents on epoxy resin adhesives. Unpublished typescript. Queen's University, Kingston, Ontario. Selwitz, C.1991. The use of epoxy resins for stone consolidation. In Material issues in art & archeology II, ed.P.Vandiver, J. R.Druzik, G. S.Wheeler, I. C.Freestone. Materials Research Society Proceedings 185. Pittsburgh: Materials Research Society. 181–91. Selwitz, C.1992. Epoxy resins in stone conservation. Research in conservation, vol. 7. Los Angeles: Getty Conservation Institute. SOURCES OF MATERIALSSikadur 35, Hi-Mod LVSika Canada, Inc., 601 Delmar Ave., Pointe Claire, Quebec H9R 4A9 Canada, (514) 697-2610 (Canada), (800) 933-7452 (U.S.) Akemi Marmorkitt 1000/Akemi Marble Filler 1000, Transparent/Clear FlowingAkemi Plastics, 1611 Hault Dr., Eaton Rapids, Mich., (800)365-8191 ext. 112 or Stone Boss, 26-04 Borough Place, Woodside, N.Y. 11377, (718) 278-2677 Hastings Plastics Co., 1704 Colorado Ave., Santa Monica, Calif. 90404, (310) 829-3449, (310) 828-6830 (fax) Cab-o-sil (Fumed Silica)Cabot Corporation, 75 State St., Boston, Mass. 02109, (617) 345-0100 Fresh white natural marbleSpada Tile Inc., P.O. Box 431, K7L4W2, Kingston, Ontario KOM 1S0 Canada, (613) 546-9373 Bolender's Ltd., P.O. Box 329, Haliburton, Ontario KOM 1S0 Canada Decayed white calcite marbleObtained by the author and originated from a broken decayed gravestone in Kingston, Ontario, Canada. Globe-o-sil “G”Hastings Plastics Co., 1704 Colorado Ave., Santa Monica, Calif. 90404, (310) 829-3449, (310) 828-6830 (fax) Microspheres 409, Low DensityGougeon Brothers, Inc., P.O. Box 908, Bay City, Mich. 48707, (517) 684-7286, (517) 684-1374 (fax) The production of this product has been discontinued. Similar products are available from: 3M Scotchlite Glass Bubbles (e.g., Glass Bubbles K37 or S32)3M Specialty Additives, 3M Center Bldg., 220-8E-04, P.O. Box 33220, St. Paul, Minn. 55144-1000, (612) 736-4133 ECCOSPHERES IG-101Emerson & Cuming Composite Materials, Inc., 59 Walpole St., Canton, Mass. 02021, (617) 821-4250, (617) 821-0737 (fax) MICA WG 160KMG Division, P.O. Box 729, Kings Mountain, N.C. 28086, (704) 739-1321, (704) 739-7888 (fax) Renaissance Micro-Crystalline WaxPi-Creator Enterprises Ltd., 44 Park View Gardens, London NW4, U.K., (44) 181-202-3435 AUTHOR INFORMATIONELEONORA E. NAGY graduated in 1984 from the Hungarian Academy of Fine Art, Budapest with an M.A. in sculpting. She then earned an M.A. in objects conservation from the Art Conservation Program at Queen's University, Kingston, Ontario, Canada. She specializes in the conservation of sculptures. She has worked as a conservator at the Conservation Center for Quebec and at the Canadian Conservation Institute, Ottawa, and as a private conservator. In 1995 she joined the Conservation Department of the Solomon R. Guggenheim Museum, where she is assistant sculpture conservator, responsible for the care of the sculpture collection and sculpture conservation-related issues of loans and exhibitions. Address: 620 W. 47th St., New York, N.Y. 10036. |