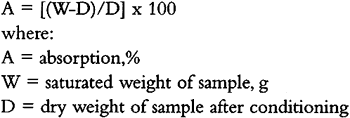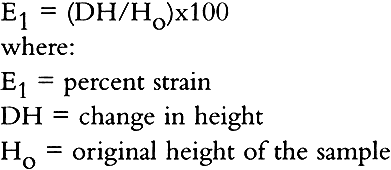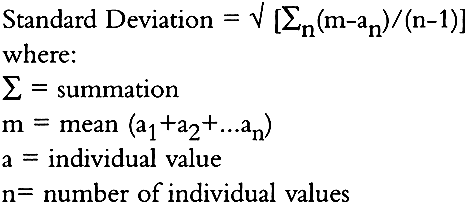FILLS FOR WHITE MARBLE: PROPERTIES OF SEVEN FILLERS AND TWO THERMOSETTING RESINSELEONORA E. NAGY
ABSTRACT—The purpose of this article is to evaluate thermosetting resin-based fills for compensation of white marble and similar stones in the outdoor environment. The article describes seven commercially available fillers, tested in two thermosetting resin formulations for structural fill on dense, whitish, translucent marble and similar calcerous stones.The influence of the concentration, color, refractive index, density, particle size and shape, and microstructure of each filler on the color, translucency, texture, density, elasticity, plasticity, reversibility, water sensitivity, and working properties of their composites will be described. A total of 187 samples were tested for density, water absorption, color, and stress-strain at break. Results of the tests show that fillers, when used in high concentration, will significantly influence the visual and mechanical properties of composites. The properties of glass microballoons (Microspheres and Eccospheres), marble dust, and Globe-o-sil fillers were found to be preferable to those of calcium carbonate, mica, and Cab-o-sil fillers. The most promising results were obtained using the hollow, glass sphere fillers (Microspheres, Eccospheres) in a Sikadur epoxy resin-to-filler w/v concentration range of 1–30%. TITRE—Produits de restauration pour le marbre blanc: Les proprietes de sept produits, de reparation et de deux resines thermodurcissables. R�SUM�—Le but de cet article est d'�valuer les mat�riaux de r�paration thermodurcissables � base de r�sine pour compense les pertes sur le marbre blanc et sur des pierres similaires restant � l'ext�rieur. Cette �tude d�crit sept produits disponibles sur le march� commercial, test�s dans deux formules de r�sines thermodurcissables pour les r�parations structurelles des marbres blanch�tres et translucides denses et des pierres calcareuses.L'influence de la concentration de la couleur, de l'indice de r�fraction, de la densit�, de la taille et de la forme des particules et de la microstructure de chaque produit sur la couleur, la translucidit�, la texture, la densit�, l'�lasticit�, la plasticit�, la r�versibilit�, la sensibilit� � l'eau et leurs propri�t�s actives seront d�crits. Un total de 187 �chantillons ont �t� test�s pour mesurer la densit�, l'absorption de l'eau, la couleur et la tol�rance � la cassure. Les r�sultats du test montrent que ces produits de r�paration, utilis� � haute concentration, influenceront, d'une mani�re tr�s marqu�e, les propri�t�s visuelles et m�caniques des compos�s. Les propri�t�s des produits de restauration de microballons en verre (Microsph�res et Eccosph�res), de la poussi�re de marbre et de Globe-o-sil �taient pr�f�rables � ceux de calcium carbonate, du mica et du Cab-o-sil. Les r�sultats les plus prometteurs furent obtenus avec les sph�res creuses en verre (Microsph�res, Eccosph�res) dans une r�sine �poxyde Sikadur une p/v d'une concentration de l'ordre de 1 � 30%. TITULO—Materiales para relleno faltantes de m�rmol blanco: Caracteristicas de siete llenadores y dos resinas de termosetting. RESUMEN—La intenci�n de este papel es evaluar rellenos basadas de resinas termosetting por compensaci�n de m�rmol blanco y piedras similares al aire libre. El papel describe siete llenadores disponibles comercialmentes, probaron en dos formulaciones de la resina del termosetting por relleno estructural en denso, blanquecino, m�rmol transl�cido y piedras similares del calc�reo.La influencia de la concentraci�n, color, �ndice de refracci�n, densidad, dimensiones y forma de la part�cula, y microstructura de cada relleno en el color, translucidad, textura, densidad, elasticidad, plasticidad, 1 INTRODUCTION1.1 SELECTION OF PROPERTIES AND METHODS FOR TESTINGFillers have been used primarily as extenders, and the full range of their benefits has not been extensively explored. This article investigates how properties of fillers such as shape, size, color, microstructure, density, and translucency affect the appearance (color, texture, translucency) and physical properties (density, hardness, elasticity, plasticity, mechanical reversibility, water sensitivity) of their composites. The evaluation of these properties is addressed for fills intended to compensate white translucent marble or similar stones (alabaster, limestones) in the outdoor environment. It is hoped that the conclusions of this study will provide some guidance for the use of fillers in sculpture and architectural conservation. To determine important parameters and testing methods in relation to optimal fills for whitish translucent calcareous stones exposed to the outdoor environment, the following properties are considered: density, water sensitivity, color, refractive index, elasticity, and plasticity. Three kinds of samples were used: prepared composite samples with various fillers; two different pure thermosetting resins; and natural marble samples. The latter two were used as controls. Testing methods listed below were carried out on a total of 187 samples. 1.1.1 DensityThe density of a fill should be close to or less than that of the original material. This density permits reversibility of the fill by mechanical means and has a potentially faster rate of deterioration than the original stone. A possible direct correlation between the density of the filler and its resulting composite was also investigated. 1.1.2 Water SensitivityThe water absorption and drying rate of a fill should be close to that of the object, so that a wet fill does not introduce moisture and therefore potential decay in the original stone. A fill should also follow the changing hues of the wet-dry cycle of the object. Although a 48-hour water immersion test is extreme relative to normal ambient external conditions on stone surfaces, such a test will indicate comparative wetting and drying patterns of stones and fills. Considering time restrictions and the relatively small size and number of samples in this study, immersion may also draw attention to possible long-term weathering effects sooner than a capillary rise procedure. 1.1.3 Color and TextureAn acceptable color and texture match between the fill and the surrounding stone is an important requirement for camouflaged stone repairs. Evaluation of texture was accomplished empirically. Color matching was rated by both empirical and instrumental methods. 1.1.4 Refractive IndexTranslucency is one of the most difficult requirements when matching white marble. On 1.1.5 Elastic and Plastic BehaviorA fill should allow expansion and contraction of the repaired stone without damaging the original material, thereby making the fill mechanically reversible. Should both materials be exposed to the same range of strain (deformation) and stress (compressive force), once the force is released, the fill may show some signs of permanent plastic deformation sooner (enter its plastic deformation range at lesser stress) than does the stone, which would still return fully to its original state (elastic deformation). To assess elastic and plastic behavior, each composite and control was tested for compressive strength and percent strain at break. Composites were sought that exhibited lower compressive strength and similar or greater strain at break (“longer” performance in the plastic region) as compared to the original stone (i.e., natural marble). 1.2 SELECTION OF MATERIALS AND THEIR COMBINATIONThermosetting resins have considerably greater strength and hardness than other resins used in conservation, making their use for structural repairs of stone very common (Larson 1978; Domaslowski 1988; Barclay and Mathias 1989; Nagy 1991; Naud� 1991; Domaslowski 1995; Technology Organization 1995;). This was the primary factor for testing thermosetting resins here. Two stone adhesives, Sikadur 35 Hi-Mod LV epoxy and Akemi Marmorkitt 1000 Transparent/Clear Flowing polyester resins, were chosen for testing. Both were developed for exterior use and are widely used in architectural and sculpture conservation. Seven fillers—calcium carbonate, Cab-o-sil, mica, marble dust, Globe-o-sil, Microspheres, and Eccospheres—were chosen because they are white or translucent and are inert. Both resins were combined with each of the seven fillers, and each resin was mixed with a filler in 3, 4, or 5 different concentrations. These composite concentrations are listed in tables 1–4, where each concentration represents the mean of 3–5 samples. To the total number of 173 composite samples, add 8 natural marble specimens (4 fresh and 4 decayed) and 6 pure resin samples (3 of each resin) that were used as controls, making the total number of samples used in this study 187. TABLE 1. EFFECT OF FILLERS ON THE DENSITY OF RESINS TABLE 2. PERCENTAGE OF WATER ABSORPTION AND DRYING RATE TABLE 3. AVERAGE CIELAB MEASUREMENTS TABLE 4. EFFECT OF FILLERS ON THE COMPRESSIVE STRENGTH OF THE PURE RESIN Pigments, typically used as coloring agents, may be considered as fillers when used in large volumes. However, the investigation of pigments as fillers is not included in this article. 2 MATERIALS AND SAMPLE PREPARATION2.1 SOURCES OF MATERIALS AND THEIR DATAMaterials were provided directly by their manufacturers. Fresh natural calcite marble and marble dust were obtained from a commercial distributor (see Sources of Materials). The decayed marble originated from a broken gravestone. Particle sizes for each material were measured by the author. Refractive indices for the two thermosetting resins were kindly provided by Christopher W. McGlinchey. All other information was obtained through literature or communication with the manufacturer. Sikadur 35, Hi-Mod LV—A two-component, solvent-free, very low viscosity (375 centipoise [cps]), high-strength, multipurpose epoxy resin adhesive. It has a clear amber color and a pot life of about 25–30 minutes. The full cure of the resin is close to complete in 72 hours but absolutely complete only in 7 days. The resin-to-hardener mixing ratio is 2:1. The RI of the cured resin is 1.567. Akemi Marmorkitt 1000/Akemi Marble Filler 1000 Transparent/Clear Flowing—A water-clear, 680 cps viscosity, two-component polyester adhesive, specifically produced as an adhesive for stone (“for Carrara marble, Ajax, Onyx translucent, and Greek Thassos marble”). It has a pot life of approximately 7–10 minutes and a full cure time of 48 hours (Akemi Plastics 1988). The resin-to-hardener mixing ratio is 100:3, and the cured resin has an RI of 1.525. Calcium Carbonate (Marble-fil) 151C—A white, low-cost, high-loading filler. This precipitated type of calcium carbonate has the finest and most uniform particle size of all the fillers that were tested. The diameter of the anisotropic, rounded hexagonal, rhombohedral particles is between 0.5–1.5 microns (μ). The RI is between 1.47 to 1.69 (mean 1.66), loose density 2.0 g/cm3. Cab-o-sil (fumed silica)—An anisotropic, white, extremely lightweight filler containing 99.9% silicon dioxide. It is practically free of contaminating metallic salts. The rounded, rough-surfaced agglomerates range from 0.5–120μ in size, the average being ca. 40μ. The mean RI is 1.54 (1.47–74), loose density 0.03 g/cm3. Wet-ground Mica 160—A grayish brown mineral additive (H2KAl3[SiO4]3) with a solid, delaminated, rounded rectangular, transparent flake structure. The average flake size is 6–10μ. The smallest flakes are 1μ; the largest particles are 30μ in diameter. The birefringent particles have refractive indices of 1.552, 1.582, and 1.588. The loose density is approximately 0.3 g/cm3. Marble Dust—One of the most popular resin additives for marble fills. The average size of the block-shaped particles is about 250μ, with a large particle size distribution ranging from approximately 20 to 800μ. The average RI of the birefringent particles is 1.65. Loose density 1.65 g/cm3. Globe-o-sil G—Has a white granular appearance. The neutral pH, low-density additive contains 71–75% silicon dioxide. The foamed particles (1–80μ) enclose cells that are separated by thin, glassy membranes. When mixed with resins, the enclosed air is held and sealed within the glassy membranes. The anisotropic particles tend to form lumps (ca. 60μ) that make up the average size particles. The mean RI is 1.45, loose density 0.11 g/cm3. Microspheres 409—A brand name for glass microballoons, which are hollow sodium borosilicate microspheres with a white powdery appearance. The particle size is 1–140μ, with an average of 50μ. The product contains some broken particles and impurities. The particles are isotropic, and the RI is 1.52. The loose density is 0.09 g/cm3. Eccospheres IG 101—Hollow sodium borosilicate glass spheres from 5–150μ in diameter. The average size of this type of glass microballon is 55μ. The RI of the isotropic spheres is 1.52. The loose density of Eccospheres is 0.17 g/cm3. The major difference between Microspheres and Eccospheres filler is that the latter is a more refined product and does not contain broken spheres or impurities. (Note that some birefringence is present in both Microspheres and Eccospheres due to the enclosed air in the hollow spheres.)
Fresh Natural Marble Specimens and Decayed Marble Specimens (4 each)—Used as a control for the duration of the tests. X-ray diffraction of the fresh natural marble specimen was performed at the Miller Museum of Geology (Badham 1991), and the results matched the mineral calcite almost perfectly. Both fresh and decayed marble specimens had a whitish color with very faint grayish veins. The decayed marble had a sugary, slightly discolored, grayish crust formation from outdoor exposure 2.2 SAMPLE PREPARATIONAll natural marble samples and controls were cut and prepared by the author. For marble dust, a piece of the fresh calcite marble was crushed using a Jaw Crusher (Braun Chipmunk VD67) and a Bico Pulverizer (disk mill by Bico, Inc.) at the Geology Department of Queen's University, Kingston, Canada. Composites and pure resin samples were made using a Plexiglas lattice open at the bottom and the top. Components of the resins were mixed according to the manufacturer's instructions. Then the filler was added, and the mixture was poured or pressed into the cells of the lattice. Renaissance microcrystalline wax was used as a separating agent. Samples for a given concentration were usually made in a single batch. The samples were left to cure for one week, then pressed out from the lattice by hand. The surfaces of the samples were sanded using 80 and 120 carat electroplated finishing sandpaper. The only exception was the sugary crust on the decayed marble. This weathered surface was not sanded and was used later for test purposes. The final size of all samples tested was 25.4 � 50.8 � 14.3 mm (1 � 2 � 9/16 in.). Unexpected mixing difficulties with a number of fillers occasionally resulted in slightly different filler-to-resin concentrations. These concentrations remained fairly similar and are still comparable. Materials for composites and controls were stored in a laboratory environment for 14 days at 73 � 4�F (23 � 2�C) at 50% � 2 relative humidity before sample preparation. The samples remained in these conditions for the duration of the tests. Samples are described as weight-volume percent (w/v): weight of resin (gram)/volume of filler (ml) � 100. A low percentage concentration indicates small amount of resin with a large amount of filler in the composite. Each datum provided is the mean of a minimum of three readings for each of the three samples. 3 RESULTS3.1 WORKING PROPERTIES OF THE MATERIALS TESTEDBoth adhesives exhibited color change during mixing and curing. The “clear amber” pure Sikadur resin turned pink immediately upon mixing, pure Akemi turned greenish. As the curing proceeded the pure Sikadur gradually changed to a clear amber and the Akemi resin to a cool clear white hue, indicating that they were close to setting. None of the fillers appeared to affect the setting time of the resins. Noticeable shrinkage of about 1 mm in length (longitudinal direction) occurred with all three pure Akemi samples. Mixtures of approximately 40% concentration (40% resin content) or greater had properties of very viscous fluids for all samples. At about 30% concentration or less, two characteristic types of behavior were noted:
Marble dust did not strictly conform to either type of behavior, but it seemed to behave more similarly to the second group of fillers. The large difference in the resin-to-hardener ratio (100:3) of the Akemi polyester resin required more precision in mixing and resulted in increased preparation error as compared to the Sikadur resin. The 7–10 minutes pot life of the Akemi resin was found to be too short for uniform distribution of high filler content in the resin. These mixing difficulties were more relevant when used with the “difficult fillers,” calcium carbonate, mica, and Cab-o-sil. Stratification of resin concentration in the mixtures during or after preparation was not noticed in this study. This fact may be due to several factors, such as the use of relatively filler-rich mixtures, a resin viscosity not low enough to easily flow in response to gravity, the relatively fast pot life of the resins, the specific attention paid to proper mixing, or a combination of all these factors. Although there was an empirically noticeable difference between the viscosities of the two resins used in the experiment, the viscosity of the pure resin did not seem to influence the working properties of the mixtures. 3.2 VISUAL OBSERVATIONSThe generally white or off-white fillers yielded a range of colors in the composites, from brilliant white to yellow, gray, and brown. Composites of Microspheres and Eccospheres were the whitest samples after curing. Some of these samples looked whiter than the natural stone controls. Cab-o-sil yielded bluish green composites in Akemi and yellow in Sikadur resin. Marble dust and Globe-o-sil appeared slightly gray in both resins, the latter being more distinct. Calcium carbonate was beige and mica distinctly brown in both resins. None of the calcium carbonate, Cab-o-sil, or mica samples exhibited any resemblance to white marble. The color of the composite was determined by the color and concentration of the filler. The color of the resin had a negligible influence. Only in cases of approximately 30–40% or higher concentration did the color of the resin become significant. In these resin-rich mixtures the amber color of the epoxy resin added a slight warm hue, while the clear transparent Akemi resin tended to modify the color of the composites to a cool blue-green tint. This was especially obvious for the samples made with Cab-o-sil and marble dust. Fillers with only one refractive index, which matches the RI of the resin, were the most transparent. As glasses and isotropic crystals satisfied this condition, composites of Microspheres and Eccospheres resembled the most translucent white marble. The rest of the fillers were not uniform crystals, so the result was generally one of graying when the average refractive index was close to that of the resin. The greater the RI difference between the filler and the resin, the greater the opacity of the composite became. Samples with an RI difference of only 0.05 between an isotropic filler and the resin exhibited increased transparency, when samples were observed side-by-side with the naked eye. Around this value, the isotropic filler/resin composites compared very favorably to the translucency of the natural marble specimens. This was the case with all glass microballoon (Microspheres With filler-rich samples (below 30% resin concentration) the texture of the composite was most similar to marble when the particle size and shape of the filler was closest to the grain of the stone. The filler-rich composites of Microspheres and Eccospheres most resembled white marble; these were followed by the samples made with marble dust. The Globe-o-sil composites resembled the decayed “sugary” marble surface. The calcium carbonate, mica, and Cab-o-sil samples, all materials with small particle sizes, had an artificially smooth, densely packed, and dull opaque appearance resembling a plastic substance rather than natural stone. 3.3 DENSITYValues obtained for density are shown in table 1. The influence of the fillers on the density of the composites was the same in both resins. Calcium carbonate, Cab-o-sil, mica, and marble dust fillers increased the density of the samples. Globe-o-sil, Microspheres, and Eccospheres radically decreased the density of the samples. (Some composites of these fillers had densities so low that they floated on water.) The density of the filler did not seem to be strictly related to the density of the resulting composite. For example, the extremely low-density Cab-o-sil filler increased the density of the samples, and they sank immediately when immersed in water. Ball-shaped or rounded filler with air content in the particles decreased the density of the composite. 3.4 WATER ABSORPTIONAs seen in table 2, a large number of Akemi resin-based composites disintegrated or were damaged by water during the absorption test. Akemi/Microspheres (6%, 20%, 26%) and The percentage weight gain of the samples, as compared to their original dry weight, is shown in table 2. As expected, the composites showed the greatest absorption of water, followed by the decayed marble, fresh marble specimens, and the pure resin samples. The drying rate of the samples is indicated in columns 4–10 of table 2. Dramatic moisture loss for all samples occurred within the first 6 hours of drying. Pure Sikadur epoxy samples dried the fastest (6 hours). The two types of natural marble specimens returned to their original dry weight in 6–12 hours. These were followed by the pure Akemi polyester samples, which dried in 26 hours. All composites took longer to dry than the stone samples, and some retained a small amount of moisture for 72 hours. In terms of the drying rate of the composites, two groups could be established.
3.5 COLORIMETRYTable 3 illustrates the mean of the readings displayed on the colorimeter. The L∗ readings show that the two pure resins were the darkest of the samples; the difference between Akemi and Sikadur was subtle though visually perceptible. The Akemi resin was somewhat on the coolish, blue-green side, and the Sikadur resin was on the warmer side. The pure resin samples were significantly darker than both the fresh and decayed natural stone samples. The fresh marble sample was slightly lighter and less yellow than the decayed marble sample. Increased concentrations of fillers increased the lightness (L∗) of the composites. The degree of this increase in lightness could be divided into two groups.
As shown in the last column of table 3, all wet natural marble controls darkened about 10% compared to their dry state. The wet composites decreased in lightness only 0–5%. Varying the concentrations of a filler did not seem to have any decreasing effect on the L∗ values of a wet sample. This decrease appeared to be filler-specific and not concentration-specific for a composite. 3.6 COMPRESSIVE STRENGTHAs seen in table 4, the range of values obtained for compressive strength was approximately 100 to 17,000 pounds per square inch (psi). Both pure resin samples had significantly greater compressive strengths (approximately 11,000–17,000 psi) than any of the marble controls tested (4,000 and 10,000 psi). Fillers either increased or decreased the compressive strengths of the pure resins and could be categorized in two basic types:
4 DISCUSSION: EFFECT OF FILLERS ON COMPOSITESThe addition of fillers influenced all visual and physical properties of the composites investigated. These properties could also be adjusted by varying the concentration of the filler. In filler-rich composites, the appearance and physical characteristics of the composites (color, viscosity, compressive strength, etc.) are determined by the fill material. A large amount of filler (30% resin concentration or less) will result in a composite increasingly similar to that filler. In resin-rich mixtures (above 30% concentration), the properties of the pure resin will determine the properties (including reversibility), regardless of the fill material. It may also be concluded that when choosing a thermosetting resin for high-filler-load composite fill, an adhesive with a long pot life (approximately 30 minutes or more) is preferable. When translucency is desired, it is advised to choose a resin with an RI as close as possible to that of the marble (approximately 1.65). 4.1 DENSITYContrary to what is expected, the density of the filler does not directly determine the density of the composite. Instead, the microstructure of the filler should be looked at when density of the composite is considered. Fillers with hollow sphere microstructure decrease the density of the resulting composite, while fillers with solid particles increase it. 4.2 WATER SENSITIVITYResults of the absorption test showed that hollow microstructure fillers in filler-rich composites (below 30% resin concentration) seem to encourage slow drying by retaining a small amount of water for an extended period of time (72 hours) as compared to the drying rate of natural stones (6 hours). There may be too much filler in the composite to form a continuous network of resin around the filler particles, so voids in the resin may retain some water upon drying. Alternatively, water may penetrate the hollow space inside the balloon particle of the filler, which remains trapped for a longer period of time. It should be noted that filler-rich, hollow spheres-based composites will continue to dry up to 66 hours after the complete drying of a natural stone, therefore keeping the original stone moist. Although, according to the test results, the retained moisture in the hollow sphere-based composites is relatively small, for A possible explanation of the disintegrated or water-damaged samples is the very short pot life of the specific Akemi resin used, which may not have permitted proper mixing. This supposition is supported by the fact that composites of comparable concentration did not disintegrate when used in the Sikadur resin. Subsequent to the completition of this study Akemi Plastics has increased the gel time of the “clear flowing” grade polyester from 7–10 to 15 minutes. This change in the product also suggests that the importance of allowing sufficient working time for thorough mixing must not be underestimated. As the disintegration phenomenon was characteristic only in the most filler-rich samples with the Akemi resin, it is conceivable that the amount of resin available to bind the large amount of particles may have been insufficient. Some of the filler particles may have been bound only by the mechanical lock of their shape within the body of the composite sample. Such flaws in these filler-rich composites may have remained unnoticed until the water immersion test when the water penetrated and forced these mechanical locks between the particles apart. The drastic results of the immersion suggest that a water immersion test may be a simple and useful qualifying test for predicting weathering performance of composites intended for exposure in an outdoor environment. The author's impression is that a capillary rise test, if performed, might not have shown disintegration of such samples so distinctly. The described water retention and disintegration of some samples indicate that problems caused by moisture might occur if composites with similar undetected flaws are used outdoors. Results of the drying rates suggest that most composites tested will likely appear as lighter areas on moist stone surfaces. 4.3 TRANSLUCENCY AND COLORAnisotropic (birefringent) substances did not lend translucency to a fill. For achieving translucency of a fill similar to that of white marble, isotropic fillers with an RI difference of 0.05 or less between the filler and the embedding resin were found to be preferable. As stated earlier, composites of anisotropic fillers will remain arble does. As all concentrations made of these fillers showed similar properties in both resins, it can be concluded that composites of calcium carbonate, mica, and Cab-o-sil fillers are of excessive compressive strength as compared to natural marble, and therefore are not recommended for compensation of decayed calcerous stones. The explanation of this phenomenon is likely to be found in the solid nature of these particles. The very small size (i.e., 0.5–8μ), rounded shape, and relatively even particle size distribution of these fills are possible contributing factors. The other group of fillers tested—Microspheres, Eccospheres, marble dust, and Globe-o-sil—particularly in concentrations between 1% and 35%, yield composites that exhibit significantly lower compressive strength (and longer performance in the plastic region) than that of natural calcareous stones and are good candidates for fills. As opposed to the other group, particles of these fillers are hollow (Microspheres, Eccospheres, and Globe-o-sil) or irregularly shaped with relatively large particle size range (crushed marble dust). These results suggest that for mechanically reversible composite fills, fillers with hollow or irregularly shaped particles with a large range of particle size, should be preferred. 5 CONCLUSIONSIsotropic fillers with hollow structures in Sikadur epoxy (with resin/filler w/v concentration from 1% to 30%) provided the most promising results for matching whitish translucent calcareous stones. The glass sphere fillers (Microspheres and Eccospheres) tested here Calcium carbonate, mica, and Cab-o-sil fillers do not appear to be appropriate choices for fills of white translucent calcareous stones. Their significant difference in color and texture from a natural stone and their lack of translucency proves them to be inappropriate for matching fills for white marble. Composites of these fillers tend to produce greater compressive strengths than the stone, rendering these fills irreversible and more likely to cause damage to original stone. This study did not investigate problems such as aging of thermosetting resins, yellowing (Down 1986), thermal expansion, resistance to freeze-thaw cycles, and biodeterioration, all of which could be topics for further study. ACKNOWLEDGEMENTSThe author would like to thank the Art Conservation Program, Queen's University, Kingston, Ontario, Canada for its support in carrying out the tests and Christopher W. McGlinchey, associate research chemist in the Paintings Conservation Department of the Metropolitan Museum of Art, New York, for kindly providing RI data for the thermosetting resins. APPENDIX1 APPENDIX: ANALYTICAL PROCEDURES1.1.1 DensityThe dimensions of the samples were measured with a metric dial caliper, 0.05 mm–130 mm, Type 6910. The samples were weighed on a Model 333 Acculab electronic digital scale with a minimum sensitivity of 0.1g. The density of the samples was calculated in grams per cubic centimeter (g/cm3). 1.1.2 Water SensitivityAll dry, conditioned samples and controls were weighed (as described in section 1.1), then immersed in a distilled-water bath using one plastic screen on the bottom and one on the top of each sample. The screen was necessary to weigh down the extremely lightweight samples of Globe-o-sil, Microspheres, and Eccospheres (these floated on top of the water) and allow sufficient absorption of water from the bottom of the dish. After 48 hours of immersion, the samples were removed from the bath, dried by blotting with a dry cloth, and then immediately weighed to the nearest 0.0001 g. For this purpose a Mettler H20T balance with a weight indication of 0.01 mg to 160 g was used. The specimens were weighed in the same order every time, at intervals of 6, 12, 19, 26, 33, 48, and 72 hours after immersion. The samples were left on a plastic lattice to air dry. A sample was considered dry when three successive measurements yielded the same reading. The first of these readings is indicated as dry state. The percentage absorption was calculated using a standard absorption formula for water (ASTM 1995a).
1.1.3 ColorThe colorimetry of the visible spectrum was carried out on a Macbeth Series 1500 Spectrophotometer using the CIE L∗a∗b∗ 1976 color system (ASTM 1995b). CIELAB 1976 color space is an analytical color measurement system for comparing or reproducing colors. In this system the scales on the three orthogonal axes (L∗ lightness, a∗ green-red, b∗ blue-yellow) are relatively uniform in comparison to other chromatic systems. Pulsed-xenon CIE65 was used as illumination with average daylight 6500�K illuminants, which included UV. A white standard (No. 051025) was used for calibration. The middle area of the largest surface was tested on each sample. The instrument displayed all L∗,a∗,b∗, and DE∗ values. Wet colorimetry samples were immersed as described in section 1.1. Readings were obtained using the spectrometer as described above. 1.1.4 Stress-Strain Behavior (Elasticity and Plasticity)Most of the samples were tested using a Unite-O-Matic compressive testing machine with a load of 0.00002–10,000 lbs. For samples of 1.0% concentration, a Wykeham Farrance Compressive machine equipped with a millivolt reader was used. Compressive strength and strain tests were conducted at the Engineering Department at Queen's University, Kingston, Canada. The samples “stood” in the machine in order to have the long dimension of the samples parallel to the axis of the compressive force. This meant that in the case of the natural stone samples the compressive force was parallel to the bedding. Compressive strengths were calculated using the standard formulas (ASTM 1995c).
The maximum speed of the loading head was 0.05 in./min. for the first and 0.045 in./min. for the second machine. Readings were taken at every 5 or 10/1000 of an inch load movement, depending on the strength of the sample. The percent strain was calculated as follows:
2 STANDARD DEVIATIONStandard deviation values were calculated as follows:
REFERENCESAkemi Plastics, Inc.1988. Material safety data sheet and physical and mechanical properties of the polyester-based products, no. 45-R0338. January 31. ASTM. 1995a. Standard test method for absorption of chemical-resistant mortars, grouts and monolithic surfacing, C 413-94. In Annual book of ASTM standards, vol. 6.05. Philadelphia: American Society for Testing and Materials. ASTM. 1995b. Standard test method for calculation of color differences from instrumentally measured color coordinates, D 2244-93. In Annual book of ASTM standards, vol. 6.01. Philadelphia: American Society for Testing and Materials.
Badham, M.1991. X-ray identification of marble. X-ray identification report of the calcite marble specimen. Kingston, Ontario: Miller Museum of Geology, Queen's University. Barclay, R., and C.Mathias. 1989. An epoxy/microballoon mixture for gap filling in wooden objects. Journal of the American Institute for Conservation28: 31–42. Domaslowski, W.1988. Thermosetting resins as used in stone conservation. In The deterioration and conservation of stone: Notes from the international Venetian course on stone restoration, ed.L.Lazzarini and R.Pieper. Rome: UNESCO. 329–49. Domaslowski, W.1995. Technology of epoxy mortars at low temperature and high relative humidity. Zabytkoznawstwo I konserwatorstwo15 (189): 33–63. Down, J. L.1986. The yellowing of epoxy resin adhesives: Report on high-intensity light aging. Studies in Conservation31:159–70. Larson, J.1979. The conservation of alabaster monuments in churches. Conservator2 (28):20–25. Naud�, V.1991. Report on the discussion about stone. AIC Objects Specialty Group postprints. American Institute for Conservation Annual Meeting, Albuquerque, N. M., June 8. Washington, D.C.: AIC. 1:111–12. Technology Organization, Inc.1995. Natural stone roundup: Guide to sources and fabrications of natural stones and to suppliers of products for their care/conservation. Technology and Conservation12 (3): 20–22. Tennent, N. H,.and J. H.Townsend. 1984. The significance of the refractive index of adhesives for glass repair. In Adhesives and consolidants, ed.N.Bromelle, E. M.Pye, P.Smith, and G.Thomson. London: IIC. 205–8. FURTHER READINGASTM. 1995c. Standard test method for compressive strength of chemical-resistant mortars, grouts, monolithic surfacing and polymer concretes, C 579-91. In Annual book of ASTM standards, vol. 4.05. Philadelphia: American Society for Testing and Materials. Brill, T. B.1980. Light: Its interaction with art and antiquities. New York: Plenum. Clifton, J. R.1984. Laboratory evaluation of stone consolidants. In Adhesives and consolidants, ed.N.Bromelle, E. M.Pye, P.Smith, and G.Thomson. London: IIC.151–55. Grattan, D. W., and R. L.Barclay. 1988. A study of gap-fillers for wooden objects. Studies in Conservation33:71–86. Nagy, E. E.1991. Effects of inorganic fillers on two thermosetting resins: Fills for deteriorated white marble. Unpublished typescript. Queen's University, Kingston, Ontario. Remillard, F.1984. The effect of solvents on epoxy resin adhesives. Unpublished typescript. Queen's University, Kingston, Ontario. Selwitz, C.1991. The use of epoxy resins for stone consolidation. In Material issues in art & archeology II, ed.P.Vandiver, J. R.Druzik, G. S.Wheeler, I. C.Freestone. Materials Research Society Proceedings 185. Pittsburgh: Materials Research Society. 181–91. Selwitz, C.1992. Epoxy resins in stone conservation. Research in conservation, vol. 7. Los Angeles: Getty Conservation Institute. SOURCES OF MATERIALSSikadur 35, Hi-Mod LVSika Canada, Inc., 601 Delmar Ave., Pointe Claire, Quebec H9R 4A9 Canada, (514) 697-2610 (Canada), (800) 933-7452 (U.S.) Akemi Marmorkitt 1000/Akemi Marble Filler 1000, Transparent/Clear FlowingAkemi Plastics, 1611 Hault Dr., Eaton Rapids, Mich., (800)365-8191 ext. 112 or Stone Boss, 26-04 Borough Place, Woodside, N.Y. 11377, (718) 278-2677 Hastings Plastics Co., 1704 Colorado Ave., Santa Monica, Calif. 90404, (310) 829-3449, (310) 828-6830 (fax) Cab-o-sil (Fumed Silica)Cabot Corporation, 75 State St., Boston, Mass. 02109, (617) 345-0100 Fresh white natural marbleSpada Tile Inc., P.O. Box 431, K7L4W2, Kingston, Ontario KOM 1S0 Canada, (613) 546-9373 Bolender's Ltd., P.O. Box 329, Haliburton, Ontario KOM 1S0 Canada Decayed white calcite marbleObtained by the author and originated from a broken decayed gravestone in Kingston, Ontario, Canada. Globe-o-sil “G”Hastings Plastics Co., 1704 Colorado Ave., Santa Monica, Calif. 90404, (310) 829-3449, (310) 828-6830 (fax) Microspheres 409, Low DensityGougeon Brothers, Inc., P.O. Box 908, Bay City, Mich. 48707, (517) 684-7286, (517) 684-1374 (fax) The production of this product has been discontinued. Similar products are available from: 3M Scotchlite Glass Bubbles (e.g., Glass Bubbles K37 or S32)3M Specialty Additives, 3M Center Bldg., 220-8E-04, P.O. Box 33220, St. Paul, Minn. 55144-1000, (612) 736-4133 ECCOSPHERES IG-101Emerson & Cuming Composite Materials, Inc., 59 Walpole St., Canton, Mass. 02021, (617) 821-4250, (617) 821-0737 (fax) MICA WG 160KMG Division, P.O. Box 729, Kings Mountain, N.C. 28086, (704) 739-1321, (704) 739-7888 (fax) Renaissance Micro-Crystalline WaxPi-Creator Enterprises Ltd., 44 Park View Gardens, London NW4, U.K., (44) 181-202-3435 AUTHOR INFORMATIONELEONORA E. NAGY graduated in 1984 from the Hungarian Academy of Fine Art, Budapest with an M.A. in sculpting. She then earned an M.A. in objects conservation from the Art Conservation Program at Queen's University, Kingston, Ontario, Canada. She specializes in the conservation of sculptures. She has worked as a conservator at the Conservation Center for Quebec and at the Canadian Conservation Institute, Ottawa, and as a private conservator. In 1995 she joined the Conservation Department of the Solomon R. Guggenheim Museum, where she is assistant sculpture conservator, responsible for the care of the sculpture collection and sculpture conservation-related issues of loans and exhibitions. Address: 620 W. 47th St., New York, N.Y. 10036.
 Section Index Section Index |