A NEW TECHNIQUE FOR DETERMINING THE DEPTH OF PENETRATION OF CONSOLIDANTS INTO LIMESTONE USING IODINE VAPORRAKESH KUMAR, & WILLIAM S. GINELL
ABSTRACT—In evaluating the effectiveness of consolidants for limestone, difficulty is encountered in the determination of the depth of penetration of consolidants. A simple procedure using iodine vapor for visualizing penetration depth is described. The technique is compared with other methods, such as fluorescent dye indicators, elemental analysis, scanning electron microscopy (SEM) observation, charring in an inert atmosphere, and acid etching, for effectiveness, applicability, and suitability. TITRE—Une Nouvelle Technique � la Vapeur de Teinture d'Iode Pour D�terminer La Profondeur de la P�n�tration des Consolidants dans le Calcaire. R�SUM�—Dans l'�valuation de l'efficacit� des consolidants du calcaire, on rencontre des difficult�s pour d�terminer la profondeur de leur p�n�tration. Une proc�dure simple � la vapeur de teinture d'iode a �t� d�velopp�e pour visualiser cette profondeur. Cette technique est compar�e � d'autres m�thodes telles que les indicateurs � base de colorants fluorescents, l'analyse �l�mentaire, l'observation au microscope �lectronique � balayage, la carbonisation en atmosph�re inerte et l'application d'acide, afin de juger de son efficacit�, de son applicabilit� et de sa convenance. TITULO—Nueva t�cnica para determinar la profundidad de penetraci�n de los consolidantes para piedra utilizando vapor iodado. RESUMEN—Cuando se eval�a la efectividad de los consolidantes para piedra caliza, se encuentran dificultades para determinar su profundidad de penetraci�n. Se ha desarrollado un procedimiento simple utilizando vapor iodado para visualizar esa profundidad de penetraci�n. Para establecer la efectividad, aplicabilidad y adecuaci�n de la t�cnica, se la compara con otros m�todos tales como: indicadores de anilina fluorescente, an�lisis de los elementos, observaciones en el microscopio de barrido electr�nico, carbonizaci�n en una atm�sfera inerte y grabado �cido. 1 INTRODUCTIONThe restoration and conservation of stone monuments and objects often involve impregnation with stone consolidating materials. However, to assess the effectiveness of such treatments, the migration and distribution of the materials inside the stone must be determined. The methods of measurement that have been investigated in the past have not been widely used in technical investigations and conservation practice due mainly to the need for sophisticated instrumentation or to their ineffectiveness in the case of some consolidants. Iodine vapor is often used in the field of thin-layer chromatography for identifying organic compounds. It is physically adsorbed on the surface of many organic compounds and produces a yellow or light brown color. The colored complex that is formed is generally reversible, leaving the substance unchanged chemically after dissociation of the adsorption complex and evaporation of the iodine. Owing to this characteristic of iodine vapor, it was thought that it might be possible to visualize the location of consolidants in stone if similar physical adsorption of the iodine vapor occurs on the polymers that are frequently used for the preservation of stone. Since iodine vapor is not adsorbed and The technique described here can be easily and rapidly carried out, and it appears to solve many of the problems associated with visualizing the location of consolidants in limestone and probably other types of porous stone as well. 2 MATERIALS2.1 CONSOLIDANTSThe following solutions, emulsions, and dispersions were tested as consolidants in varying concentrations:
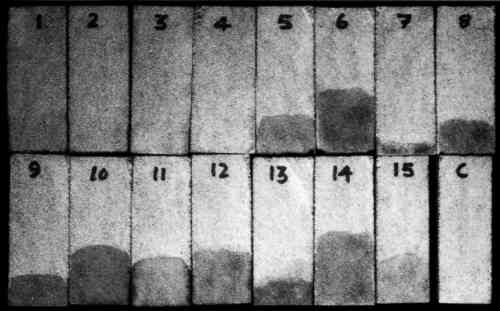
2.2 STONEIndiana and Xunantunich limestones were used for this study. Indiana limestone is a beige-colored, homogeneous, fossiliferous limestone. Chemically and mineralogically it is almost pure calcite with a small amount of quartz, hematite, and magnesium. The porosity of this limestone is ∼18.6 %. The Xunantunich (Belize) limestone is of marine origin and is inhomogeneous. Chemically and mineralogically it is a fine-grained, almost pure, calcite with minor amounts of magnesium, iron, aluminum, and silica impurities (<2%). The hardness varies with the distribution of soft and hard calcite. The porosity of this type of stone, as measured by mercury intrusion porosimetry, varies from 26 to 56%. 2.3 IODINE VAPORIodine vapor was generated by room temperature sublimation of solid iodine in a closed glass chamber. Solid iodine and iodine vapor are hazardous materials and should be handled with care and in accord with Materials Safety Data Sheet (MSDS) recommendations. 3 METHOD3.1 CONSOLIDATION TREATMENTSamples of both limestones, in the form of 5 cm cubes, were immersed in the respective solutions of the consolidants to a depth of 5 mm and were impregnated by capillary rise. After 10 minutes of impregnation, samples were removed and allowed to cure at room temperature for 15 days. All consolidants mentioned above were applied on Indiana limestone. Only a limited number, however, were tested on the Xunantunich limestone. 3.2 DETERMINATION OF PENETRATION DEPTHAfter the solvents had evaporated and the consolidants had cured, samples were sectioned parallel to the direction of capillary rise and placed on a perforated Teflon base in a glass chamber within a fume hood. All samples were In separate tests, cured consolidant films, limestone dust, and both consolidated and unconsolidated limestone were exposed separately to iodine vapor, and it was found that only the consolidant films and the consolidant-treated stone developed color. It is important to note here that exposure time should be slightly longer for some consolidant concentrations. If a consolidant is not completely cured when the test is performed, it is possible that the yellow color may persist for a longer period. However, after a longer exposure to an open-air environment, the color will eventually disappear. If the iodine treatment is performed before solvent evaporation is complete, iodine reaction with the solvent may occur and the solvent penetration depth, rather than that of the consolidant, may be indicated. 4 RESULTSThe measured depth of penetration of the various consolidants into the two types of limestones is shown in tables 1 and 2. Figure 1 shows some of the Indiana limestone samples after iodine exposure. TABLE 1 DEPTH OF PENETRATION OF CONSOLIDANTS INTO INDIANA LIMESTONE TABLE 2 DEPTH OF PENETRATION OF CONSOLIDANTS INTO INDIANA LIMESTONE
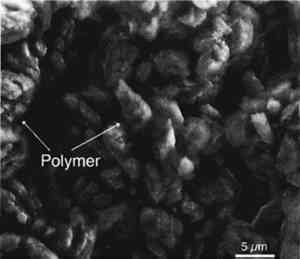
The samples were examined carefully in the environmental scanning electron microscope to
5 COMPARISON WITH OTHER METHODSThe iodine vapor technique was compared with a number of other possible methods to assess its effectiveness, applicability, and suitability for determining the penetration depth of consolidants into limestone. The same consolidants, concentrations, and stones were used for the purpose of comparison. 5.1 FLUORESCENT DYE TECHNIQUEThe fluorescent dye technique has been used as a possible staining method for the identification and localization of proteins and other binding media in conservation science (Talbott 1982; Wolbers and Landrey 1987). A high luminosity fluorescence probe has also been used to visualize restoration materials (Golikov and Zharikova 1990). However, its effectiveness has not been reported on a wide range of materials. Of the available fluorescent dyes, an aqueous solution of Rhodamine B is commonly used and was tested for this application. Except for the epoxy-treated sample, no fluorescence was observed under ultraviolet illumination. Practically, the dye does not seem to be effective for the other consolidants. Moreover, it permanently stains the sample, therefore limiting its use for other analyses. 5.2 ELEMENTAL ANALYSISElemental analysis provides information on the distribution of characteristic elements in the consolidant that are not present in the stone. Analysis of serial sections of the stone for these elements should indicate the penetration depth. In the case of epoxy or polyurethane polymers, such an element would be nitrogen. However, because of the complexity and expense of this method, it has not proven to be a practical or popular procedure for determining the migration and distribution of consolidants in stone. It has also been suggested that electron beam microprobe analysis for the metallic elements contained in some polymerization catalysts (e.g., lead and tin) could be used to determine the location of polymers. This method was tried on a tin-catalyzed alkoxysilane consolidant in limestone but was unsuccessful owing to the low tin concentration. 5.3 SEM EXAMINATIONFreshly fractured surfaces of consolidated limestones were examined in the environmental scanning electron microscope (ESEM), and secondary electron imaging was used to locate the consolidant. Previous experience with these samples had shown that the consolidant could be morphologically distinguished from the limestone. Since the transition from the consolidant-rich to consolidant-free areas takes place over several millimeters, it proved difficult to estimate the depth of consolidant penetration exactly by examining fractured sections in the ESEM. Also, because the ESEM in its environmental mode has a lower magnification limit of 100–200x, the survey of large areas of topographically complex surfaces is difficult because the field of view is limited. Examination of polished sections might provide a method of quantifying the amount of consolidant present (using image analysis) provided sufficient contrast exists between the mounting medium and the consolidant. This technique is suitable for determining the migration and distribution of consolidants in stone but requires expensive instrumentation and a high level of skill. 5.4 CHARRING TECHNIQUEIt was thought that some organic polymers in the limestone samples would char at high temperatures, and the black charred component could be observed visually. Several limestone samples that had been treated with a variety of consolidants were charred in a nitrogen environment at 400�C. Both the treated and untreated areas became darker without any differentiation, and thus the charred organic polymer could not be visualized separately. From this experiment, it appeared that the charring method is neither suitable nor convincing for 5.5 ACID ETCHINGThe acid etching procedure has been used to determine the resin distribution in the pores of stone (Domaslowski 1988). This method involves etching stone slices with a 5% solution of hydrochloric acid for �, 1, or 2 hours. Calcium carbonate is decomposed in the zones where consolidant is absent. Several consolidated stone samples were tested using this method, but it was not effective on the Indiana limestone or on the stones containing less than 3% by weight of consolidant. Both the consolidated and unconsolidated zones of Xunantunich limestone dissolved completely. This method seems to be effective only on pure limestone having consolidant concentrations greater than 5%. 5.6 WETTABILITY TESTA simple test for determining the location of hydrophobic materials is to wet the cross section with a solution of a water-soluble dye. The test is effective only when the consolidant being tested is hydrophobic. If the treatment with a nonhydrophobic consolidant rendered the treated area nonporous, the dye solution would not penetrate but would remain on the surface of the stone, and this area would appear to be hydrophobic. In this case, the method would work. 6 CONCENTRATION DEPENDENCE OF COLORTo determine if the color intensity shown in the iodine vapor technique is proportional to concentration of consolidants, two different concentrations of each consolidant were applied to the surface of limestone samples by brushing on two bands that were separated by untreated stone. These samples were then kept in an iodine vapor chamber for 5–10 minutes along
These variations in color can serve as a qualitative indicator for determining the unknown concentration distribution of the same consolidant in stone. It is necessary that the stone material for preparing such references be the same stone in which the concentration profile of a consolidant treatment is to be determined. 7 DISCUSSION AND CONCLUSIONSCompared to the other possible methods described, the iodine vapor technique seems to be superior in practical use for determining depth of penetration of a consolidant into porous stone. The test can be performed easily in the laboratory or in the field, and it seems to be effective for a variety of consolidants currently used in the conservation of stone. For maximum effectiveness, the stone to be tested should be light in color, and the consolidant concentration in the stone should be at least 1% by weight. Investigation has shown that the intensity of color is dependent, qualitatively, on the concentration of the consolidant in stone, as well as the depth of penetration, and therefore the distribution of the consolidant in the stone can be determined by the iodine vapor technique. It may also be used for estimating the distribution of a consolidant in stone after aging in the natural environment by using an unaged reference distribution pattern of that consolidant (Kumar and Ginell 1995). This application may be very important for assessing the long-term effectiveness of any consolidant. Finally, as the iodine vapor technique does not require sophisticated instrumentation, it is an economical test procedure that can be used by most conservators. The experimental observations reported here reveal that the iodine vapor technique is rapid, simple to use, reliable, inexpensive, and more accurate for determining consolidant penetration depth and distribution in stone than methods currently in use. ACKNOWLEDGEMENTSThe authors would like to acknowledge with gratitude the assistance of Eric Doehne for ESEM studies and Luiz Souza, Mary Striegel, Michele Derrick, and Charles Selwitz for their helpful advice. SOURCES OF MATERIALSRhoplex AC-33, Rhoplex AC-630, and Acryloid B-72Rohm & Haas, Independence Mall West, Philadelphia, Pa. 19105 El Rey Superior 200El Rey Stucco, 4100 Broadway SE, Albuquerque, N.M. 87105 Carboset 514-HB. F. Goodrich, Speciality Polymers and Chemicals Division, 9911 Brecksville Rd., Cleveland, Ohio 44141–3247 ERL-4221Union Carbide and Plastics, Solvents and Coatings Materials Division, 39 Old Ridgebury Rd., Danbury, Conn. 06817 Eponex 1510Shell Oil, PO Box 4320, Houston, Tex. 77210 Jeffamine T403Huntsman, P. O. Box 219, Conroe, Tex. 77305 Bayer, 100 Bayer Rd., Pittsburgh, Pa. 15205 Conservare SS-OH and Conservare SS-HProSoCo., P.O. Box 171677, Kansas City, Kans. 66117 PVA (AYAF)Union Carbide, 100 Ocean Gate, Long Beach, Calif. 90802 Iodine crystalsAldrich Chemical, 1001 W. Saint Paul Ave., Milwaukee, Wisc. 53233 REFERENCESDomaslowski, W.1988. The mechanism of polymer migration in porous stones. Wiener Berichte �ber Naturwissenschaft in der Kunst4–5:402–25. Golikov, V. P., and Z. F.Zharikova. 1990. Analysis of spatial distribution of hydrophobic restoration materials by means of fluorescent probes. ICOM Committee for Conservation preprints, 9th Triennial Meeting, Dresden, Germany. Paris: ICOM. 1:29–32. Kumar, R., and W. S.Ginell. 1995. Evaluation of consolidants and biocides for limestone in tropical environments. Getty Conservation Institute Scientific Program, Los Angeles. Talbott, R. R.1982. The fluorescent antibody technique in the identification of protinaceous materials. In Papers presented by conservation students at the Third Annual Conference of Art Conservation Training Programs. Kingston, Ontario: Queen's University. 140–49. Wolbers, R., and G.Landrey. 1987. The use of direct reactive fluorescent dyes for the characterization of binding media in cross-sectional examinations. AIC preprints, American Institute for Conservation 15th Annual Meeting, Vancouver, British Columbia. Washington, D.C.: AIC. 168–202. AUTHOR INFORMATIONRAKESH KUMAR received his M.Sc. (1984) and Ph.D. (1989) in organic chemistry from the University of Gorakhpur, India. Since 1997, he has been working as a research associate with the Materials Research Program of the National Center for Preservation Technology and Training. Prior to this he was a conservation scientist (fellow) in the Monuments and Sites Conservation Research section of the Getty Conservation Institute. He worked previously as an academic visitor, during 1991 and 1992, at the Institute of Archaeology, University College, London, and from 1987 to 1992 as an assistant chemist in the Science Branch of Archaeological Survey of India. During past years, his research has primarily been focused on the development of new treatments for the conservation of stone monuments and archaeological sites in tropical regions. However, he has also been intimately involved with many other aspects such as preservation of museum objects and site management. His current research interests are in the development and evaluation of consolidants and biocides for stone conservation. Address: NCPTT, NSU Box 5682, Natchitoches, La. 71497. WILLIAM S. GINELL received his Ph.D. in physical chemistry from the University of Wisconsin in 1949. Since then he has held research positions at Brookhaven National Laboratory, Atomics International, Aerospace Corporation, and McDonnell Douglas Astronautics Company. He joined the Getty Conservation Institute in 1984 as head of Materials Science and currently is head of Monuments and Sites Conservation Research. His principal research interests are conservation in humid tropical environments, stone conservation, seismic stabilization of historic structures, and architectural conservation. Address: Getty Conservation Institute, 1200 Getty Center Dr., Ste. 700, Los Angeles, Calif. 90049.
 Section Index Section Index |