ULTRASONIC MISTING. PART 1, EXPERIMENTS ON APPEARANCE CHANGE AND IMPROVEMENT IN BONDINGSTEFAN MICHALSKI, & CAROLE DIGNARD
2 APPEARANCE CHANGE EXPERIMENTS2.1 APPEARANCE CHANGE: MATERIALS TESTEDThe following modern pigments were tested: chrome yellow, ultramarine, ivory black, raw
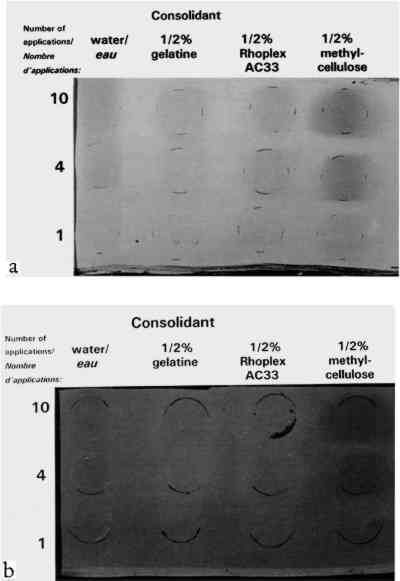
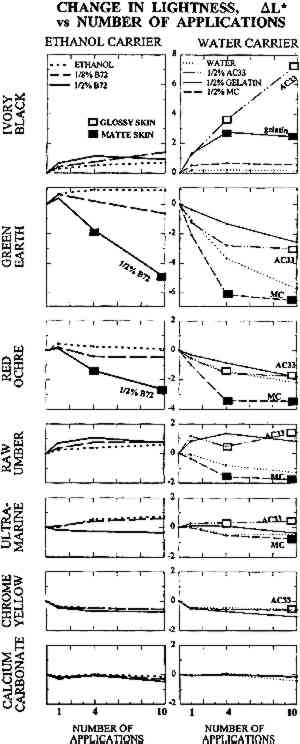
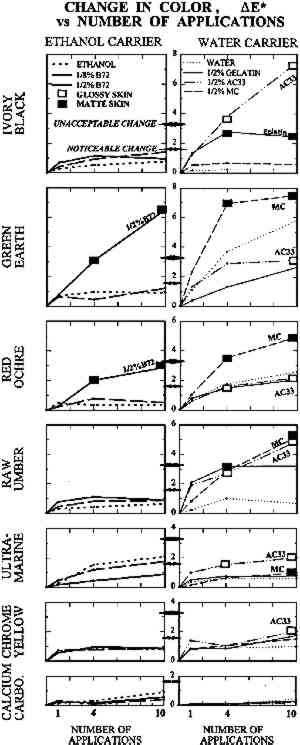
TABLE 1 THICKNESS OF PAINT FILMS ON GLASS SUBSTRATE The dried paints were cohesive compared to their bulk pigment powder, and no pigment fell away when the plates were turned upside down. Most were, however, very vulnerable to contact or abrasion damage. Thus they represented the most extreme form of powdery paints encountered in museum artifacts, such as those devoid of binder or those in which surface binder has been disintegrated by UV exposure. The solvents and solutions tested were water, ethanol (95%), gelatin in water at 0.5% w/v, Rhoplex AC-33 acrylic colloidal dispersion in water at 0.5% w/v, methylcellulose of low substitution (400 cP) in water at 0.5% w/v, and Acryloid B-72 acrylic resin in 95% ethanol at 0.5% w/v and at 0.125% w/v. Powdery paint samples were misted with 1, 4, or 10 applications of the consolidant or solvent. Using the misting technique described in the previous section, consolidant mist was applied continuously to one area of paint until the solution reached the bottom of the paint layer and flooding of the surface began, then the nozzle was moved to an adjacent area of paint. The result was a slow scanning application of mist across the paint layer, the speed determined by the imminence of flooding. Treatments did not involve precise volumes of solution; the intent was simply to apply as much consolidant as would wick in, as in practical treatments. The penetration of the solution to the bottom of the paint layer was monitored using a mirror to view the bottom of the glass plate. Samples were allowed to air-dry between applications. 2.2 APPEARANCE CHANGE: METHODS OF MEASUREMENTColor change was measured using a Minolta Chromameter model CR200 with its companion data processor unit. This instrument averages reflectance from a 1 cm diameter area. Before-treatment readings can be stored by the device, and in conjunction with after-treatment readings, a full range of conventional colorimetric parameters are tabulated. These are briefly outlined below. Probably the most familiar color system to conservators is the Munsell Color System (Billmeyer and Saltzmann 1981, plates I–III). It is derived directly from visual experience and uses three coordinates. These are hue (red, green, etc.), value (lightness), and chroma (the variation from maximum color to a gray of the same lightness, as in the chroma adjustment on television sets). The preferred color system in the scientific literature, however, is L∗a∗b∗, derived from fundamental data on the color receptors in the human eye (Billmeyer and Saltzmann 1981, plate IV). The L∗ coordinate, “lightness,” is straightforward and corresponds to the Munsell coordinate “value.” The coordinate a∗ corresponds to redness-greenness, and coordinate b∗ to blueness-yellowness. Since the L∗a∗b∗ coordinates are unfamiliar to many, it can be helpful to transform them to coordinates analogous to Munsell's: hue, H∗; lightness, L∗; and chroma, C∗. Color change can be reported as the change In this investigation, the change in lightness ΔL∗ and change in color ΔE∗ are reported. Changes in hue ΔH∗ and chroma ΔC∗ were monitored but are reported only in a few necessary instances. Lightness proved the most useful coordinate overall and corresponds to the darkening, or occasionally lightening, a conservator might expect from experience with consolidation. With optimum presentation of an optimum discrimination test, a just perceptible color difference is about 0.25–0.5 units in these scales, depending on the region of color space (Billmeyer and Saltzmann 1981, 106). The definition of “acceptable” color change has been endlessly debated in various industrial applications. In the gray scale for evaluating color change used by International Organization for Standardization (ISO) lightfastness tests, for example, the first full step in the scale, GS4, is ΔL∗ or ΔE∗ = 1.6, and the second full step, GS3, is ΔL∗ or ΔE∗ = 3.2. Purely for the sake of discussion, and recognizing that practical judgments will depend on color and circumstance, the step defined by GS4 for “noticeable” color change, ΔE∗ greater than 1.6, and GS3 for “unacceptable” color change, ΔE∗ greater than 3.2, has been adopted. “Imperceptible” color change has been reserved for ΔE∗ below 0.5. The formation of a visibly distinct consolidant layer or “skin” on or near the top of the paint was determined by eye. The boundary between treated and untreated areas of paint was viewed from the top of the paint layer and compared to the same boundary viewed from the bottom of the paint layer (the glass sample plate was turned upside down). There was no ambiguity: skins were readily apparent as a large color change above and little to no color change below. Skins were further judged as matte or glossy by holding the glass plate and tilting it freely, looking for any specular reflection of ceiling fluorescent lighting from the paint surface. As with color change interpreted as skin formation, there was no ambiguity in the gloss/matte results. A glossy skin was deemed an unacceptable change in appearance, even if the measured color change alone did not warrant this categorization. During visual inspection, we also looked for any change in surface texture between treated and untreated areas. 2.3 APPEARANCE CHANGE: RESULTSThe change in lightness, ΔL∗, is reported in figure 2, and the color difference, ΔE∗, in figure 3. The presence of a glossy skin is noted by a white square, a matte skin by a black square. No single treatment caused noticeable skinning.
Despite important practical differences among consolidants discussed later, the most important experimental variable in color change was the pigment. Results will be discussed by pigment, from the most problematic to the least. None of the pigments showed any noticeable change in texture, such as “orange peel” or raindrop effect, or a change from velvet to smooth, or a ridge of dislodged powder at the edge of the treated area. 2.3.1 Ivory BlackAC-33 and gelatin caused noticeable lightening with one application. The effect of 4 and 10 applications of gelatin was similar. Color change (lightening) remained just within the acceptable range, but the formation of a matte skin resulted in an unacceptable change in appearance. Four applications of AC-33, however, resulted in unacceptable lightening and a glossy skin. Ten applications doubled the color change. Methylcellulose did not cause noticeable change even at 10 applications. Ethanol and the B-72 solutions also did not reach noticeable change at 10 applications. 2.3.2 Green EarthThe green earth paint was the most vulnerable 2.3.3 Red OchreAs with green earth, red ochre was darkened by most treatments, except for initial slight lightening with one application of ethanol and its B-72 solutions. As with green earth, a dark skin formed with 0.5% B-72 at 4 and 10 applications, but color change remained just within the acceptable range at 10 applications. All aqueous solutions darkened the paint and caused almost noticeable gains in chroma. Only gelatin remained acceptable at 10 applications, causing less change than water alone. 2.3.4 Raw UmberSome results from the misting of raw umber with water and the aqueous consolidants are shown in figure 4b. Water alone darkened umber noticeably after four applications. Of the aqueous consolidants, gelatin and AC-33 lightened this paint, while methylcellulose darkened it. In terms of color change, AC-33 and methylcellulose became unacceptable at 10 applications, but the formation of skins at 4 applications placed them in the unacceptable appearance category. Change in chroma and hue contributed significantly to the total color change. Unlike green earth and red ochre, umber responded well to ethanol and the B-72 solutions, the noticeable change occurring on 1 application and essentially no further change after 4 and 10 applications. 2.3.5 Ultramarine and Chrome YellowOf the colored pigments, the ultramarine and chrome yellow gave the smallest color changes. Like white, they were very bright to begin with. Most darkened (except ethanol and .125% B-72 in ultramarine), but the darkening alone would have been almost imperceptible. It was the accompanying shift in hue and chroma that made total color change noticeable by 10 applications of most consolidants. Glossy skin that developed made AC-33 unacceptable at 4 and 10 applications. 2.3.6 Calcium CarbonateAlthough this white showed darkening when wetted by any of the solutions, as expected for a white pigment with poor scattering power in any binder, the darkening disappeared once the consolidant solutions dried, even at 10 applications. This finding demonstrates that whiting can be a very misleading pigment to use when testing a subtle consolidation process, because it is the least sensitive to color change. |