AQUEOUS LIGHT BLEACHING OF PAPER: COMPARISON OF CALCIUM HYDROXIDE AND MAGNESIUM BICARBONATE BATHING SOLUTIONSTERRY TROSPER SCHAEFFER, VICTORIA BLYTH-HILL, & JAMES R. DRUZIK
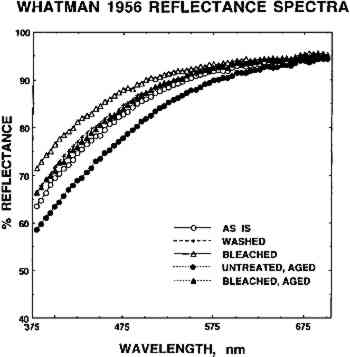
3 RESULTS3.1 SPECTROCOLORIMETRYExamples of reflectance spectra obtained for the sized artists' paper (W56) “as is,” after washing in Mg(HCO3)2 solution, and after aqueous light bleaching in Mg(HCO3)2 are shown in figure 2. The reflectances of untreated and of aqueously light bleached samples that had been artificially aged are also shown. The lighter in color a paper sample is, the larger percentage of the incident light that it reflects. Washing and aqueous light bleaching increased the reflectance of
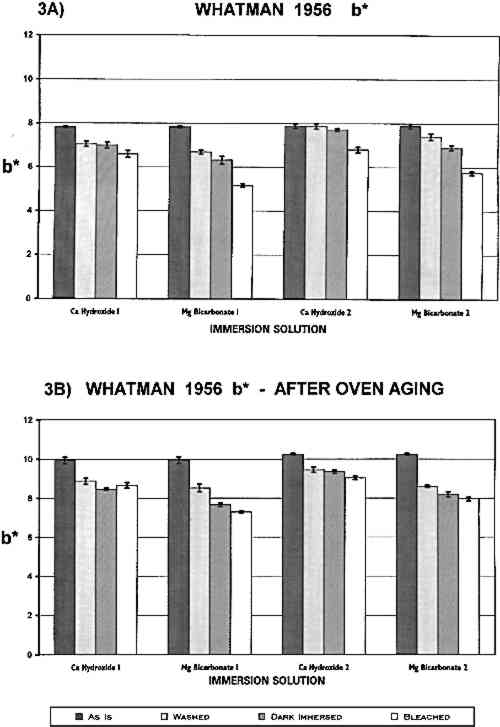
The complete colorimetric data are summarized in tables 1 and 2. It can be seen that the only treatments that caused significant changes in the unsized W1 paper (table 1) were aqueous light bleaching in the two immersion solutions, which reduced b∗ slightly. This finding may indicate that the W1 samples were less yellow after treatment, although the changes were too small to be detected by eye. (It should be borne in mind that the W1 paper looked “white” initially.) Ca(OH)2 and Mg(HCO3)2 were equally effective as immersion solutions. Humid oven aging increased the yellowness of all W1 samples (more positive b∗). The data suggest that in each experiment the samples that had been bleached in Ca(OH)2 solution may be slightly less yellow following artificial aging than the other W1 samples. Aqueous light bleaching in Ca(OH)2 and dark reimmersion in Mg(HCO3)2 made the gelatin-sized W56 paper appear slightly less yellow (table 2 and fig. 3). In both experiments, this paper was lightened somewhat more by aqueous light bleaching in Mg(HCO3)2 solution.
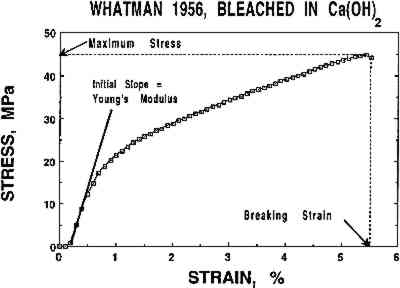
Humid oven aging consistently darkened all the W56 samples slightly (lower L∗; see table 2). In each experiment, these samples were yellowed significantly by the artificial aging process (increased b∗ in fig. 3b, cf. fig. 3a). The washed and bleached samples all remained less yellow than the untreated paper, and again in each experiment the samples that had been bleached in Mg(HCO3)2 appeared slightly less yellowed than those that were bleached in Ca(OH)2. However, it should be noted that the washed, dark reimmersed samples were not significantly more yellow after artificial aging than the corresponding bleached samples. 3.2 TENSOMETRYA plot of the stress (force per unit cross-sectional area) on a strip of paper as a function of the strain (percentage increase in length) in the paper while it is stretched to its breaking point can be used to determine several tensile properties of the paper. The three chosen for this investigation are maximum stress, which is an indicator of the strength of the paper sheet; the slope of the initial straight line portion of the curve (Young's modulus), which is a measure of the stiffness of the paper; and the breaking strain, which is inversely proportional to the brittleness of the paper and thus is a relative indication of its pliability. The stress-strain curve in figure 4 was obtained when a 0.5 in. wide strip of the W56 sample that had been washed and light-bleached in Ca(OH)2 solution was slowly stretched in the machine direction until it broke. The features described above are indicated on this graph. The same mechanical properties were evaluated for all samples. (The complete numerical data are available from the authors on request.)
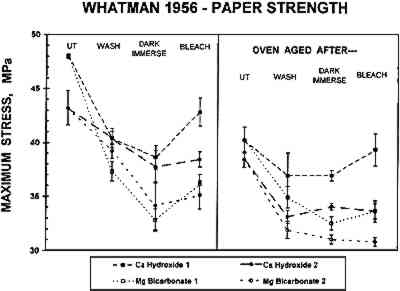
The tensile properties of the untreated papers and the samples aged without other treatments are summarized in table 3. The gelatin-sized W56 paper was more than twice as strong and pliable as the unsized W1 paper, and Young's modulus of the W56 paper was almost twice as large as that for W1. Humid oven aging of the W1 paper did not change its tensile properties significantly. However, aging did reduce the strength and pliability and increase the stiffness of the W56 paper to some extent. Bathing each of the papers decreased the strength and stiffness somewhat and increased the pliability slightly. Changes in tensile properties caused by subsequent TABLE 3 TENSILE PROPERTIES OF UNTREATED PAPERS Differences in tensile properties of either paper due to the nature of the bathing solution were mostly insignificant or not reproducible. An exception to this behavior was the maximum strength of the W56 paper (fig. 5). In each experiment (square or circular symbols) samples bathed and reimmersed in Mg(HCO3)2 (open symbols) were significantly weaker than the samples that had been treated in Ca(OH)2 (solid symbols). These differences tended to persist upon artificial aging. Exposure to light did not appear to be a determining factor in this phenomenon.
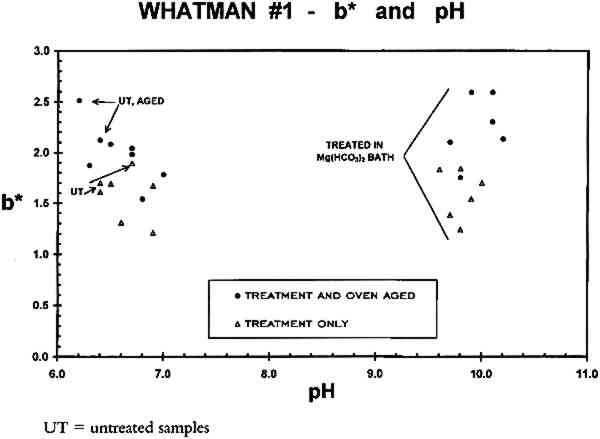
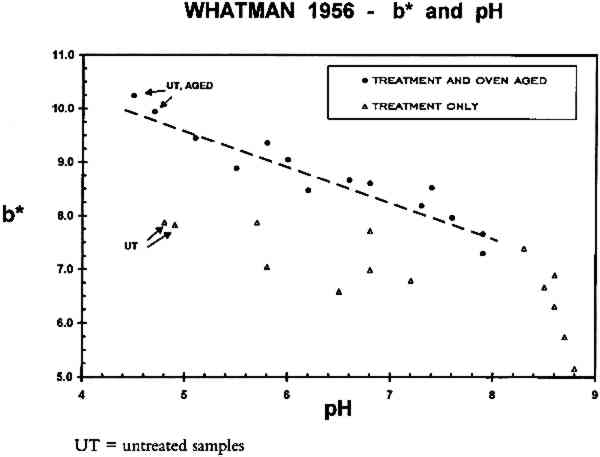
3.3 ACIDITYThe pH of the freshly diluted Ca(OH)2 solution (0.4% of saturation, or ca. 1.0 mM) was 10.1 � 0.2. This solution can neutralize acidic groups present at a concentration below 1 mM, but it cannot provide a buffer reserve once the neutralizing capacity has been used up. (The pH falls significantly as the solution sits in an open container, due to absorption of CO2 from the air. CaCO3, which has less than one-tenth the solubility of Ca(OH)2 in water, is formed and will precipitate out if the initial concentration of the hydroxide is too high.) Thus the Ca(OH)2 bathing solution is unlikely to provide a buffer reserve to acidic materials immersed in it. This effect can be seen in table 4 where the pH values, obtained by the cold extract method described in appendix B, have been listed. The procedure gives values accurate to ca. � 0.2 pH TABLE 4 COLD EXTRACT pH VALUES The Mg(HCO3)2 bathing solution is approximately 19 mM (see appendix A) and has a pH of ca. 7.0 initially. This solution has significant buffering capacity and imparts a buffer reserve to the sample papers, as can be seen from the pHs of the samples following immersion in this solution (table 4). In fact, bathing the acid-free W1 paper in Mg(HCO3)2 renders it distinctly alkaline. As a consequence, humid oven aging of these samples does not cause them to become acidic. The buffer reserve provided to the acidic W56 paper samples by two immersions in the Mg(HCO3)2 solution is also adequate to prevent these samples from becoming acidic as a consequence of artificial aging, although their pHs are lowered by about one unit. In both experiments, and for both the unsized and sized papers, the acidity of the aqueously light bleached samples is not significantly different from that of the samples that had been reimmersed in the dark. This finding was true both after initial treatment and after humid oven aging of the papers. The data indicate that exposure to light per se during immersion was without deleterious effect on this property of the paper samples. A correlation was observed between acidity and b∗ for the gelatin-sized, artificially aged paper samples, regardless of which treatment was used. This correlation is shown in figure 6, where b∗ of all W56 samples is plotted as a function of sample pH. Data from both experiments are included in this figure. The aged samples were in the humid oven for either 24 or 28 days (appendix A); it is not known whether the result would be the same for other aging times. The dashed line is the least-squares best fit (r = 0.947) to all the after-aging data points (solid circles). In contrast, there is no correlation between b∗ and paper acidity for the unsized W1 samples (fig. 7). The results of this investigation cannot supply a causal relationship for the observation, but the complete absence of a correlation for the unsized paper (fig. 7) suggests that the presence of gelatin sizing may be relevant. Barrett and Mosier (1995) recently reported correlations among lightness, pH, and gelatin content for a selected group of historic gelatin-sized papers.
3.4 GELATIN CONTENTImmersion of the heavily gelatin-sized W56 paper results in leaching of some gelatin sizing into the bath, as evidenced by the slight tendency of the solution to foam and to feel slick or sticky. To monitor this loss of sizing, the qualitative TAPPI test for glue in paper was modified for use as a semiquantitative assay (Schaeffer 1995). Some results of the application of this procedure are given in table 5, where data from at least four different determinations on samples from the two experiments have been averaged. Immersion of W56 samples in either Ca(OH)2 or Mg(HCO3)2 solution decreased the amount of gelatin in the paper, measured as percentage by weight of the analyzed sample. More gelatin was lost from the samples bathed in Mg(HCO3)2 than from those bathed in Ca(OH)2. The second immersion of the samples usually resulted in a further loss of gelatin, although not as much was lost as in the first bath. It should be noted that more than 40% of the gelatin sizing initially present in the untreated W56 paper had been removed after two immersions in the Mg(HCO3)2 solution. TABLE 5 PERCENT GELATIN BY WEIGHT OF WHATMAN 1956 SAMPLES |