AQUEOUS LIGHT BLEACHING OF PAPER: COMPARISON OF CALCIUM HYDROXIDE AND MAGNESIUM BICARBONATE BATHING SOLUTIONSTERRY TROSPER SCHAEFFER, VICTORIA BLYTH-HILL, & JAMES R. DRUZIK
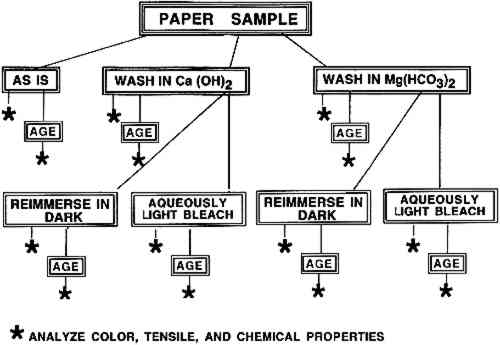
2 MATERIALS AND METHODS2.1 MATERIALSTwo papers with 100% cotton cellulose fiber were used for these experiments. The first was a heavily gelatin-sized artists' paper made by Whatman in 1956. It also contained alum. This paper, naturally aged for ca. 35 years, had been stored flat in the dark for the last several years. It was described in detail in an earlier publication (Schaeffer et al. 1992). Unsized Whatman 1 filter paper served as a control for the response of the α-cellulose fiber furnish alone to the treatments. Neither paper had disfiguring stains or noticeably uneven discolorations. The preparation of the Ca(OH)2 and Mg(HCO3)2 immersion solutions used in the treatment procedures is described in appendix A. Solutions at these or similar concentrations 2.2 EXPERIMENTAL PROTOCOLThe experimental plan was designed to permit a direct comparison of the effect of each treatment on the two different papers. The handling and conservation treatments of the paper samples were similar to those that conservators apply to works of art on paper. The procedures that mimicked conservation treatments were washing in dilute Ca(OH)2, aqueous light bleaching of the washed paper in Ca(OH)2, washing in Mg(HCO3)2, and aqueous light bleaching of the washed paper in Mg(HCO3)2. The control procedure of reimmersing a washed paper sample in fresh bathing solution in the dark was also performed to distinguish any changes due to exposure to light from those due solely to the additional immersion step. The initial washing step was included in the protocol because conservators routinely begin a treatment designed to reduce paper discoloration by bathing the object. To carry out this plan, seven samples of both the Whatman artists' paper (W56) and of the Whatman 1 paper (W1) were needed. One piece of each type of paper was reserved for analysis “as is.” Three pieces of each type were washed in trays of 0.4% (of saturation, by volume) Ca(OH)2 solution, and the remaining three pieces in 18.5 mM Mg(HCO3)2 solution (see appendix A for calculation of concentration). One of each group of three washed samples was reserved as the “washed only” sample. The remaining two samples in each group, after being dried and flattened, were reimmersed in trays of the bathing solution. While one of these was aqueously light bleached under a bank of daylight fluorescent lights, the other remained in the dark (the tray was wrapped in heavy-duty aluminum foil). Eight individual samples were treated simultaneously, four immersed in Ca(OH)2 solution (two W56 and two W1) and four immersed in Mg(HCO3)2 solution. Following this treatment, the bleached and dark-immersed samples were dried and flattened. Then every paper sample was cut in half. One half was artificially aged in a humid oven to obtain an indication of the long-term effectiveness of the different treatments. Procedural details are described in appendix A. A flow chart for the experimental procedure is shown in figure 1. The entire undertaking was repeated with different sheets of the two papers. The humid oven aging protocol was not reproduced exactly in the second experiment; details are given in appendix A. Statistical analyses of the data from each experiment were performed separately.
The effects of the various treatments on the papers were determined by objective assessment of the appearance, tensile properties, and some chemical properties of each sample. These properties were monitored after treatments and also after artificial, humid oven aging. The “as is” samples of each paper were analyzed before and after aging for comparative purposes. Appearance was assessed by reflectance spectrometry using a spectrocolorimeter. Reflectance spectra were recorded at four different places on each side of each sample. CIE L∗ a∗ b∗ values were computed by the instrument from the spectral data, and the readings were averaged. L∗ is an indication of the lightness or darkness of the sample, a∗ is a measure of the red-green value, and b∗ indicates the blueness or yellowness of the paper. The average values and population standard deviations are shown in tables 1 and 2. TABLE 1 WHATMAN 1 CIE L∗a∗b∗ COLOR VALUES TABLE 2 WHATMAN 1956 CIE L∗a∗b∗ COLOR VALUES Mechanical strength, stiffness, and brittleness of the papers were determined from features of the stress-strain curves obtained by slow elongation of 0.5 in. wide strips of each sample on a tensometer. All paper strips were mounted and stretched in the machine direction at a slow constant rate of elongation, until they broke. Four or five strips of each sample were measured, Two measures of chemical changes in the papers were used. First the pH of the paper samples was measured following the TAPPI cold extract pH method (TAPPI 1987b), but using commercial pH standards and one-half the weight of sample and volume of water called for in the published procedure. Gelatin content of the sized W56 samples was determined by a modification of the qualitative hydroxyproline assay for glue in paper published by Tappi (TAPPI 1987a). Purified photographic gelatin served as the standard for this semiquantitative procedure (Schaeffer 1995). Details of all the analytical methods are given in appendix B. |