SOME NEW ANALYTICAL TECHNIQUES FOR USE IN CONSERVATIONMICHELE R. DERRICK, ERIC F. DOEHNE, ANDREW E. PARKER, & DUSAN C. STULIK
5 PHOTO-INDUCED CHEMILUMINESCENCE (PICL)Chemiluminescence is a process in which light is emitted from a material as a direct result of the decay of an excited state species formed from a chemical reaction. Because nearly all organic materials undergo some form of oxidative degradation that results in these faint chemiluminescent emissions, measurement of the light emission has been used to monitor deterioration in organic materials. A new instrument, which measures photo-induced chemiluminescence (PICL) emissions, is being used to investigate photo-oxidative processes occurring in materials used in the construction and conservation of cultural objects (Gromek and Derrick 1993). This sensitive instrument can measure minute changes in relative degrees of oxidation of a material at levels well below the detection limit of other techniques. Its precision makes chemiluminescence an ideal method for determining evidence of sample oxidative degradation long before the deterioration is manifested in any physically measurable quantity. This evidence can then be extended to the long-term aging effects or physical changes in the material (Mendenhall 1990). In this manner chemiluminescence can be used to predict the service life of a material under near ambient conditions (George et al. 1983).
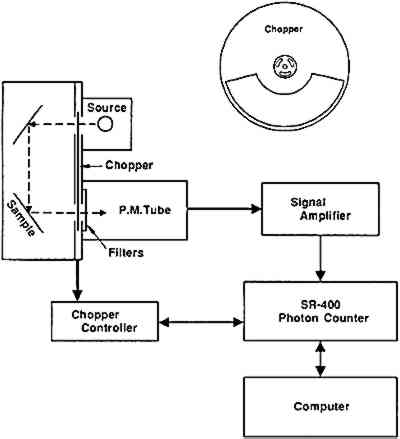
Figure 10 is a cross-sectional view of the current configuration of the PICL chamber. Extremely mild photo-oxidation is induced through the irradiation of the sample with UV light from a small penray lamp. To prevent the source light from irradiating the photomultiplier detector, the experiment is done in a pulsed manner using one chopper (fig. 10, upper right) that can alternatively block either the source or the detector. Thus, when the sample is being
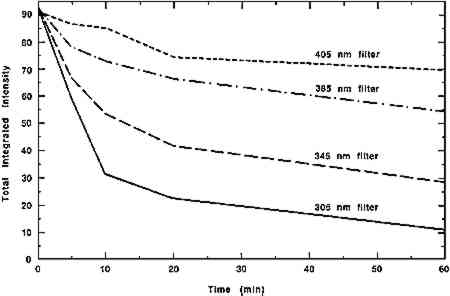
An ideal application for chemiluminescence investigation is photochemical reactions. Since it is the energy of the photons, not the number, that produces photochemical change, reactions still occur at low light levels (Feller 1964). Yet because other analytical techniques are not sensitive enough to measure changes due to low-intensity irradiance, it is sometimes assumed falsely that no reaction occurs. The effects of photographic flashes on photosensitive materials have previously been examined using multiple flash (25,000) exposures (Hanlan 1970). PICL, however, can detect changes in the oxidation states of silk and paper after exposure to a single flash. In this initial study, a sample of silk and a sample of paper were exposed to direct unfiltered light from a Sunpak 333D electronic flash. Prior to exposure an initial PICL measurement was taken. Then the sample was removed from the instrument, exposed to the flash, and placed back in the instrument for remeasurement. The flash was placed directly on the sample, and the flash duration was 1 millisecond. Table 2 presents the values for the total integrated chemiluminescent signals obtained for the samples after exposure to the indicated number of flashes. The value of the integrated signal is proportional to the amount of oxidizable species available on the surface of the sample. Thus the decrease in the measured values as the number of flashes increase indicates that the light from the flash has caused some oxidation of the sample, thereby depleting the level of oxidizable species available to produce the PICL signal. In addition to the flash measurements, a reproducibility study was done in which three PICL measurements were taken on another piece of silk over a similar TABLE 2 COMPARISONS OF PICL MEASUREMENTS FOR SILK AND PAPER EXPOSED TO AN ELECTRONIC PHOTOGRAPHIC FLASH Another area in which PICL measurements may be useful is the evaluation of real-time oxidation effects due to various illumination levels and energy distributions. As a preliminary study, four silk samples were placed under cutoff filters of 305, 345, 385, and 400 nm and exposed to a solar simulation source (Heraeus Sun Test Chamber, 30�C, approximately 100 lux) for short time periods (1 minute to 1 hour) to determine the effects of ultraviolet energy on sample oxidation. The results from the total integrated PICL emissions for each filtered exposure versus the total exposure time are plotted in figure 11. The results indicate that the greater the radiant energy exposure the silk sample receives, the more the sample is oxidized. As the wavelengths of UV energy are increasingly filtered out, the level of change is smaller. It is interesting to note, however, that some oxidation is still occurring when an ultraviolet exclusive cutoff filter of 400 nm is used. Significantly more oxidation occurs under the 385 nm filter, an illumination spectrum similar to conditions found in many museums.
PICL is a sensitive technique for measuring photo-oxidation under near ambient conditions. The low intensity light source provides, in practical terms, a nondestructive probe for the measurement of real-time oxidation. However, the instrument is still in the developmental stages, and much work remains to be done before a full understanding of the phenomenon of PICL is reached. |