SOME NEW ANALYTICAL TECHNIQUES FOR USE IN CONSERVATIONMICHELE R. DERRICK, ERIC F. DOEHNE, ANDREW E. PARKER, & DUSAN C. STULIK
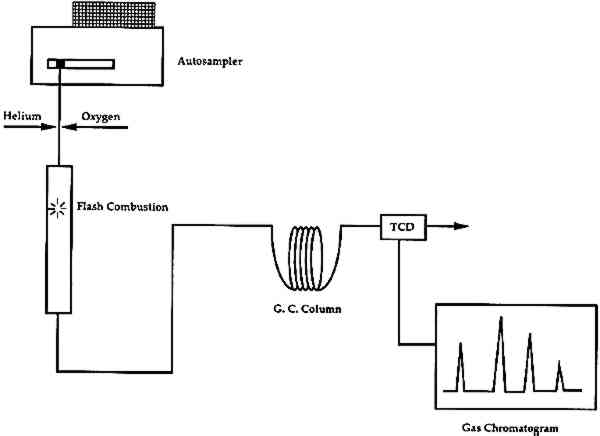
4 ORGANIC ELEMENTAL ANALYSIS (OEA or CHNS-O)Organic elemental analysis (OEA or CHNS-O) provides quantitative information on the amounts of carbon (C), hydrogen (H), nitrogen (N), sulfur (S), and oxygen (O) in a combustible material. This information can be used for the characterization of organic materials in a sample. Organic elemental analysis is not a new technique, but recent advances in instrument design and sample size requirements have resulted in a re-examination of its potential for use in analysis of art materials. Modern elemental analyzers are now capable of working with samples of approximately 100 μg (depending on the carbon content of the sample). Organic elemental analyzers combust the sample, then separate, identify, and quantify the resultant gases (Pella 1990). A schematic of the instrument is shown in figure 8. It can be divided into two main sections: (1) combustion, and (2) separation. For analysis, a sample, encased in a tin cup, is dropped vertically into an oxygen-rich chamber and is combusted into its elemental oxides at temperatures in excess of 1700�C. The combustion gases (CO2, H2O, N2, and SO2) are then transferred to a gas chromatographic (GC) column for separation. The gases are detected and quantified with a thermal conductivity detector (TCD). Analysis for oxygen in the sample requires a slightly different instrument configuration, for which the internal atmosphere and GC column have been changed. The sample is pyrolyzed in an oxygen-free atmosphere, converting the oxygen in the sample to carbon monoxide (CO), after which it is separated and measured by GC-TCD.
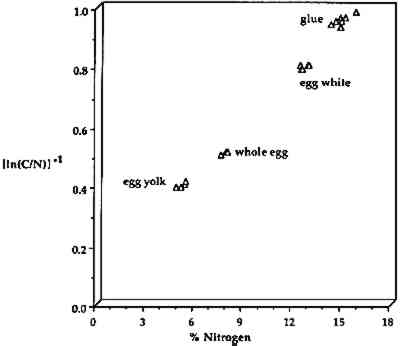
Organic elemental analyzers can detect carbon in absolute concentrations as low as 0.5 μg, making OEA useful as a confirmation technique. In the OEA identification of proteinaceous binding media, the elemental composition varies with different types of binder as shown in figure 9, where %N is plotted vs. [In (C/N)]−1 for egg yolk, whole egg, egg white, and animal glues. The types of binder are readily differentiated using this method even though the sources of materials exhibited a wide range. The eggs were from chicken and ducks, and the glue samples were from rabbit skin, fish, bone, etc. The date of origin for the samples ranged from 1934 to the present. All types of single component binders, with or without noncombustible pigments,
Another application for OEA is as a screening method for carbon-14 analysis. In one case a sample was removed from a rock art drawing at the archaeological site of Santana do Riocho in the Brazilian state of Minas Gerais. OEA analysis of the sample was performed as an initial survey to determine whether the sample contained enough carbon for carbon-14 analysis. Results showed that carbon was present in the sample in low amounts and thus a large sample would be required for carbon-14 analysis. This result was significant because OEA provided this information on a small sample (140 μg) at approximately 1/50th the cost of carbon-14 analysis. The proportions of carbon, nitrogen, and hydrogen in the sample determined that an organic material was definitely present. However, because multiple materials may be present and because the In preparation for the restoration of the tomb of King Tutankhamen, some preliminary experiments have been done to develop methods for the analysis of binders in the presence of carbonates containing minerals. Table 1 shows the results of OEA on a standard mixture of gum arabic with calcium carbonate to determine the binder-to-pigment ratio. A warm water extraction method was used to remove the soluble components in the mixture prior to analysis. The results show that the extraction technique was successful. Thus there is a potential that OEA can be developed as a method for binder-pigment analysis. TABLE 1 BINDER-TO-PIGMENT RATIOS OF GUM ARABIC TO CALCIUM CARBONATE The main advantage of organic elemental analysis is that it is readily available at almost any commercial analysis laboratory at a reasonable cost (about $20 per sample). However, because it has rarely been used for samples from cultural objects, it is important that initial studies be conducted to understand the potential and limitations of the technique. One disadvantage is that the analysis is not specific for source components and that the results are a measure of the total amount of combustible material in the sample regardless of whether it is due to one component or many. |