EFFECTS OF AGING AND SOLVENT TREATMENTS ON SOME PROPERTIES OF CONTEMPORARY TRACING PAPERSDIANNE VAN DER REYDEN, CHRISTA HOFMANN, & MARY BAKER
5 PROJECT 3: EFFECTS OF SOLVENTS AND APPLICATION TECHNIQUES ON SELECTED CONTEMPORARY TRACING PAPERS5.1 RESEARCH PROCEDURES5.1.1 Solvent Treatment MethodsAs shown in table 1, four solvents (water, ethanol, acetone, and toluene) were applied by three techniques (immersion, poultice, and suction disk) to each sample of paper using the following techniques. IMMERSION TECHNIQUE: For the immersion technique, a 150 square mm section of each sample was dipped into a 3 ml solution of solvent and held there for 3 seconds. The sample was then allowed to air-dry. POULTICE TECHNIQUE: The poultice technique consisted of placing diatomaceous earth saturated by solvent (0.3 gm diatomaceous earth to approximately 1–2 ml solvent, depending on solvent) on the front of each paper sample lying on a nonabsorbent support.2 The poultice covered an area approximately 5mm in diameter, and, contrary to normal practice, the wet poultice was not surrounded by dry poultice to diffuse the transition from wet to dry areas. The poultice was allowed to air-dry before being removed by an air bulb and brushing.
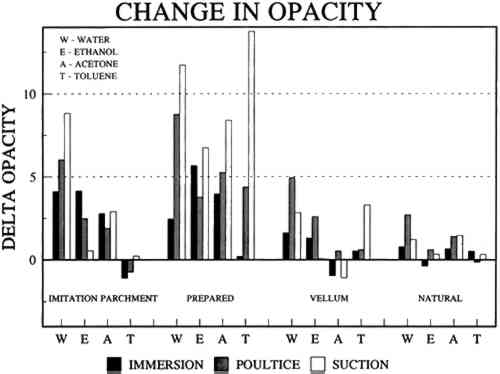
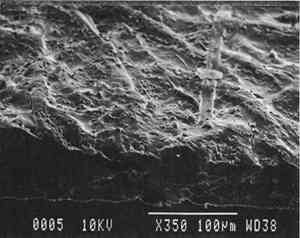
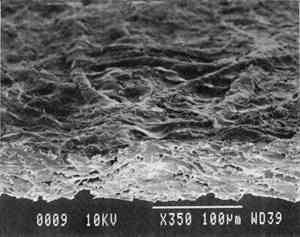
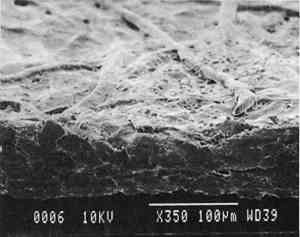
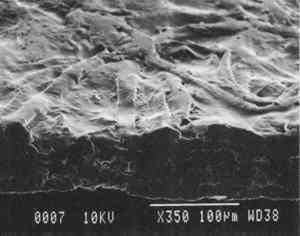
2 Diatomaceous earth (hydrated silica from diatom plant skeletons) was selected as a poultice for its working properties since, unlike gel poultices (methylcellulose, agarose, starch paste, or hydroxypropylethylcellulose) it can be mixed with aqueous or nonaqueous solvents to form a plaster or paste that absorbs solutes as it dries to a powder, which can then be brushed off. It is more cohesive than fused silica. It is whiter than Fuller's earth, which is formed from hydrated silicates of magnesium, calcium, aluminum, or other metals. It is more controllable than organic solid poultices such as powdered cellulose, paper, or cotton. SUCTION DISK TECHNIQUE: The suction disk technique consisted of applying three drops of each solvent locally by dropper on a 15 cm fritted glass bead disk (masked off with polyester film), reaching an optimum pressure of ca. 25 in Hg. 5.1.2 Measurement of PropertiesEvaluation of the effects of solvents and application techniques on tracing papers included measurement of properties (table 3), as outlined in section 3.1.3, as well as subjective observation of overall appearance (color, opacity, and gloss) in visible and ultraviolet light, and tracking dislocation of furnish materials by SEM and UV microscopy. 5.2 FINDINGS AND DISCUSSION FOR PROJECT 3Aqueous and nonaqueous solvents are used in conservation treatments of tracing papers to aid in the removal of adhesives and stains, and in humidification prior to TABLE 5 SOME PROPERTIES OF SELECTED SOLVENTS The effect of solvents on paper composition may also be influenced by how long a solvent is retained in a paper. Solvents retained in coating films may change the dimensional or chemical stability of a film. The retention time may be influenced not only by the evaporation rate but also by the solvent structure, concentration, temperature, ambient RH, and paper porosity. Depending on solvent molecular structure and shape, for example, water, ethanol, acetone, and toluene would have respectively increasing solvent retention times, since small solvent molecules that are linear, unbranched, and symmetrical (like water) can pass more easily between polymer molecules than solvents that are larger or branched. However, the speed of evaporation can reduce the normal retention time, so that, acetone for example may be removed from paper faster than water and ethanol, even though it is a larger molecule. Likewise, toluene is removed faster than water, due to its higher evaporation rate, even though it is several times larger. On the other hand, the degree and speed of solvent-substrate interaction and retention time may be manipulated by conservators. Conservators can alter this interaction by selecting and controlling any of various solvent application techniques, using immersion, poultice, or suction systems. Such manipulations alter the conditions of solvent concentration; of the direction of penetration, evacuation, and evaporation of solvent; and of time and rate of solvent exposure. During conservation treatments, for example, as a solvent volatizes or evaporates, the resultant removal of heat may lower the temperature of the surrounding area to below the dew point, causing condensation of water from the atmosphere. Water, whether introduced as condensation or as a solvent, may be absorbed into a polymer film coating and become trapped there as chemically bound water. Such alteration in the hydration state of a film may change its refractive index, and an increase in light scatter causes the film to appear lighter and consequently more opaque, a phenomenon referred to as “bloom,” sometimes seen in coatings on furniture and paintings. For instance, acetone, a hydrophilic ketone that can dissolve cellulose derivatives as well as certain resins and waxes, has a high vapor pressure, and its high evaporation rate can cool coating surfaces, causing moisture condensation leading to bloom (Hess 1965; Horie 1987). Under normal circumstances, pure ethanol is anhydrous and not likely to Under the conditions of this study, color was not significantly affected by the application of solvents. In addition, although solvents caused the opacity in most papers to increase to a degree that conservators might find visually unacceptable, the measurable percentage point change in opacity was generally less than the 3–7 percentage points for rag or the 9 percentage points for chemical wood pulp paper considered unacceptable (following aging) by the U.S. Federal Specifications for Tracing Papers (UU-P-561H 1972) (fig. 4). The major exception occurred with the prepared tracing paper, which underwent significant change in opacity (greater than 7 percentage points) following suction application of all solvents except ethanol (figs. 3b–d, 4). Water applied by suction disk caused a significant change in opacity for the chemical pulp imitation parchment. Water in fact generally caused the greatest net change in properties regardless of application technique or type of paper, resulting in appreciable planar distortions and a decrease in gloss. Ethanol decreased gloss in all but the natural tracing paper, regardless of application technique. Toluene generally appeared to have the least effect on the most properties of most of the
The increase in opacity measured in this study (fig. 4) could be caused by different reactions (i.e., chemically bound water, crazing, leaching of coating material or additives, or fiber debonding), depending on the solvent (water, ethanol, acetone, or toluene) and application technique (immersion, poultice, or suction). For example, treatment with ethanol applied by immersion or suction
The paper most affected by all solvents was the heavily coated prepared tracing sample. Only immersion in toluene had no readily apparent effect, whereas when applied by suction disk, toluene caused an increase in opacity readily apparent in visible light. This finding suggests that the effect of toluene on this paper during a treatment, 5.3 CONCLUSIONS FOR PROJECT 3The effects of solvents on the surface of tracing papers vary a great deal and seem to depend on the composition of the paper, the type of solvent, and the application technique. Of the papers studied here, the natural tracing paper was the least affected and the heavily coated prepared tracing paper was the most affected by the various solvents and application techniques. Water effected the greatest changes (increase in surface distortion and opacity and decrease in gloss) and toluene the least. The property most severely affected was opacity, which increased in most cases. Dimensional stability was most affected by water. |