PROTECTION OF ARCHIVAL MATERIALS FROM POLLUTANTS: DIFFUSION OF SULFUR DIOXIDE THROUGH BOXBOARDCHARLES M. GUTTMAN, & KENNETH L. JEWETT
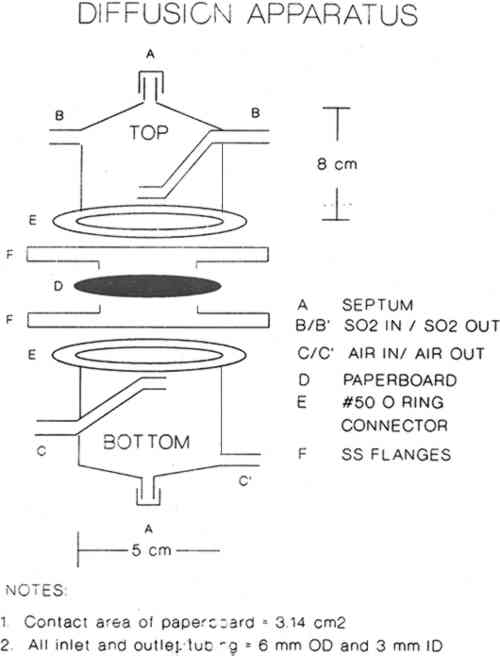
2 EXPERIMENTAL2.1 CHEMICALS AND MATERIALSCylinders of 10–500 ppm concentrations of SO2 in air were obtained from commercial sources and used as received. Calibrations and diffusion experiments were performed using those cylinders. Calibrations were also performed using SO2 gas permeation tubes. Concentrations of SO2 were accurately delivered into the sulfur-specific detector by controlling the gas flow through the permeation tubes with previously calibrated mass flow controllers. Boxboard samples were obtained from NARA and Conservation Resources International. Table 1 describes these boxboards. TABLE 1 2.2 APPARATUSDiffusion measurements were performed using the apparatus shown in figure 1. SO2 at a concentration of Ci in air was passed through the top compartment while air was passed through the bottom compartment. Both gases were prehumidified by passing them through water solutions slightly acidified with sulfuric acid and maintained at 10.5 �C. Thus these gases contained relative humidities of 54% � 5% at the measurement temperature of 20�1�C.
Sierra Instruments Model 840 flow controllers were used to control flow rates in the diffusion experiments and also to deliver accurately the desired gas flow rates when performing calibrations using SO2 gas permeation tubes. For the diffusion experiments the flow rates were varied from 25 ml/min to 100 ml/min. Sulfur dioxide concentrations were measured using a Hewlett Packard Model 5730A gas chromatograph with a sulfur-selective flame photometric detector (GC–FPD). A Supelco Chromosil 330 (⅛ in � 8 ft Teflon) column was used to verify that there were no other sulfur-containing gases present. Quantitative measurements were then made with a short length of ⅛ in diameter Teflon tubing containing no packing material. This absence of packing material ensured far greater measurement precision. When the apparatus was clamped as shown in figure 1, the unsealed boundary condition (see section 3.2) was obtained. The sealed boundary condition was obtained by additionally placing a bead of GE Clear RTV Silicone Rubber Adhesive Sealant around the circumference of the paperboard (D) seal with the stainless steel flange (F). Then all the paperboard extending beyond the lip of the flange was covered with an additional 8 mm of the sealant. The sealant was allowed to cure while gas flowed on both sides of the sample. On curing, the sealant gives off acetic acid. High concentrations of acetic acid did not affect the magnitude of the sulfur dioxide signal in the GC-FPD. |