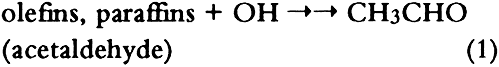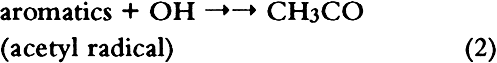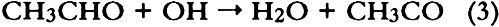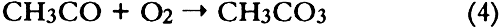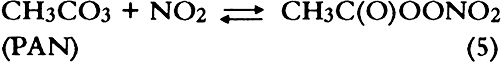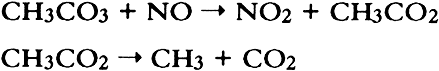EXPOSURE OF ARTISTS' COLORANTS TO PEROXYACETYL NITRATEEDWIN L. WILLIAMS, ERIC GROSJEAN, & DANIEL GROSJEAN
1 INTRODUCTIONAir pollutants such as sulfur dioxide and ozone have been recognized as a threat to cultural property for a number of years (Thompson 1978; Shaver et al. 1983; Baer and Banks 1985; Brimblecombe 1990). More recently, detailed surveys of air pollutant levels inside museums and of the corresponding indoor/outdoor concentration ratio have been carried out (Druzik et al. 1990; Hisham and Grosjean 1991a, 1991b). These studies have shown that a number of air pollutants—including ozone, nitrogen dioxide, peroxyacetyl nitrate, nitric acid, formaldehyde, and chlorinated hydrocarbons such as methyl chloroform and tetrachloro-ethylene—are frequently present in museum air. These studies have also brought into focus the need to assess possible damage resulting from exposure of works of art to air pollutants. Indeed, a number of artists' colorants have been shown to fade substantially when exposed to levels of ozone relevant to urban air quality (Shaver et al. 1983; Drisko et al. 1985; Whitmore et al. 1987; Whitmore and Cass 1988). Photochemical oxidants that are formed, along with ozone, in polluted urban air include nitrogen dioxide (NO2), nitric acid (HNO3), and peroxyacetyl nitrate (PAN, CH3C(O)OONO2). The effects of NO2 and nitric acid on colorants have been recently studied (Whitmore and Cass 1989; Salmon and Cass 1991; Grosjean et al. 1992). Thus, of the major photochemical oxidants that are nearly always present in polluted urban air, only PAN remains to be investigated for its possible threat to colorants and other materials relevant to museum collections. While air pollution scientists have studied PAN for more than 30 years, it may be less familiar to the art conservation community. Therefore, a brief summary of the properties of PAN relevant to this study is given here. PAN is the first and most abundant member of a family of compounds, the peroxyacyl nitrates (RC(O)OONO2, R = alkyl group), which were not known to chemists until they were identified in urban smog in the early 1960s (Stephens 1969). PAN and other peroxyacyl nitrates have no known direct sources and are therefore excellent indicators of photochemical pollution. They are formed in situ in the atmosphere in complex sets of reactions involving hydrocarbons (paraffins, olefins, aromatics) and oxides of nitrogen emitted by stationary (e.g., power plants or oil refineries) and mobile sources (e.g., automobiles, buses, or aircraft). These reactions are initiated by the hydroxyl radical (OH) and lead to aldehydes, which in turn react with OH and with nitrogen dioxide to form PAN and its homologues, e.g.:
Once formed in the atmosphere, PAN and other peroxyacyl nitrates may be removed by dry deposition to surfaces and by physical and chemical processes. Thermal decomposition, the reverse reaction in equilibrium (5) above, increases rapidly with increasing temperature. Thus, the atmospheric persistence of peroxyacyl nitrates is limited by their thermal stability. It is also limited in the presence of nitric oxide (a major air pollutant emitted by mobile and stationary sources) because of the rapid reaction between nitric oxide and the peroxyacyl radical formed in reaction (4) above:
PAN and other peroxyacyl nitrates are mutagens, eye irritants, and phytotoxins (Peak and Belser 1969; Taylor 1969; Stephens 1969; Temple and Taylor 1983; Shepson et al. 1986). They are strong oxidants: like ozone, they oxidize potassium iodide, and this reaction serves as a basis for a widely used method to measure “total oxidants” in polluted air. PAN also oxidizes amino acids (Mudd 1966). While several of the adverse properties listed above may be directly relevant to objects of artistic or historical value, there is no information available regarding the effects of PAN and other peroxyacyl nitrates on materials. Peroxyacyl nitrate has been identified in urban air since the mid-1960s (Stephens 1969); its concentrations may reach 30–50 ppb during smog episodes (Stephens 1969; Grosjean 1984; Williams and Grosjean 1990). Thus, PAN is often the second most abundant oxidant (after ozone) in polluted ambient air. While PAN has been most often observed in the Los Angeles, California area (Williams and Grosjean 1990 and references therein), it is also present in all urban areas of the world that experience photochemical smog, including Rome, Mexico City, Cairo, Athens, and Rio de Janeiro (Tanner et al. 1988; Lalas et al. 1987; G�sten et al. 1988; Tsani-Bazaca et al. 1988; Nasralla and Shakour 1981; Grosjean et al. 1990). Several of these urban areas also have a high density of cultural property. While PAN is predominantly an outdoor pollutant, it has been observed inside public buildings (Thompson et al. 1973) including museums (Hisham and Grosjean 1991a, 1991b) at concentrations comparable to outdoor levels. In addition, computer simulations suggest that PAN may form indoors in well-lit buildings including museums (Nazaroff and Cass 1986) by chemical reactions involving NO2 and reactive volatile organics including acetaldehyde. Both nitrogen dioxide and acetaldehyde are frequently present in indoor air (Yocom 1982; Crump and Gardiner 1989) and have been observed in museum air in California (Hisham and Grosjean 1991a, 1991b) and in Brazil (Grosjean et al. 1990). In this article, we describe the methods and findings of an investigation focusing on color changes resulting from exposure of 31 artists' colorants to levels of PAN relevant to museum air quality. This study, while limited in scope to colorants, represents the first attempt to address the issue of possible PAN-induced damage to museum collections. The implications of our results with respect to PAN-induced damage are briefly discussed. The observed color changes are compared to those obtained for the same colorants exposed to other air pollutants, including ozone (Whitmore and Cass 1988), nitrogen dioxide (Whitmore and Cass 1989), nitric acid (Salmon and Cass 1991), and formaldehyde (Williams et al. 1992). |