CORROSION MECHANISMS FOR COPPER AND SILVER OBJECTS IN NEAR-SURFACE ENVIRONMENTS
MICHAEL B. McNEIL, & BRENDA J. LITTLE
3 MICROBIOLOGICALLY INFLUENCED CORROSION
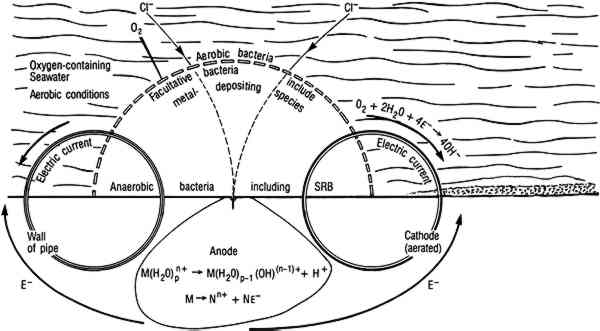
In natural environments bacteria attach to solids, including metals, where they colonize the surface and produce biofilms. Micro-organisms within the biofilm are capable of maintaining an environment at the surface of the artifact that is radically different from the bulk in terms of pH, dissolved oxygen, and other organic and inorganic species (fig. 5). In some cases, these interfacial conditions cannot be maintained in the bulk medium at room temperature near atmospheric pressure. For example, obligate anaerobic SRB that can only grow in anaerobic environments are routinely isolated from surfaces in oxygenated environments. SRB are a diverse group of bacteria that can be isolated from many environments, but their principal habitats are soil, sediments, and seawater (Miller and Tiller 1970; Battersby 1988). In natural environments SRB grow in consortia with oxygen-using microorganisms that can deplete the local environment, driving the conditions at the biofilm surface interface toward more negative Eh values and providing conditions for SRB growth. SRB are frequently isolated in association with tubercles formed by metal-depositing bacteria (Pope 1986). Within biofilms and tubercles SRB produce high concentrations of sulfides, acid-producing bacteria shift the interfacial pH (Postgate 1979; Little et al. 1990), and chloride ions collect to maintain electroneutrality (Pope 1985)(fig. 6). Microbiological effects are important to the corrosion behavior of silver, copper, and their alloys in natural environments.
Fig. 5.
Possible reactions within a naturally occurring biofilm
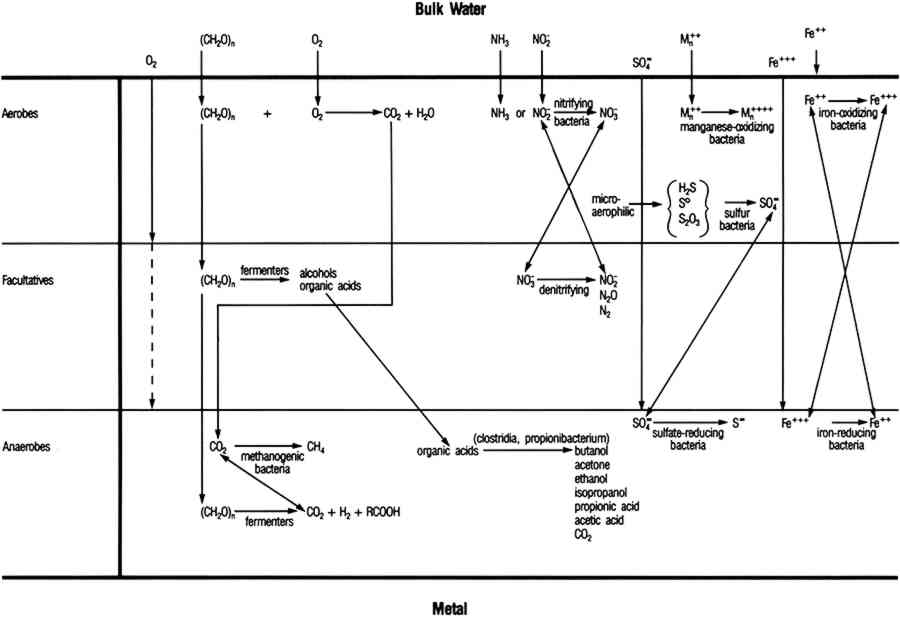 |
Fig. 6.
Typical reactions associated with bacterial deposits on metal surfaces
 |
|

