AN INVESTIGATION INTO THE REMOVAL OF ENZYMES FROM PAPER FOLLOWING CONSERVATION TREATMENTTHERESA MEYER ANDREWS, WILLIAM W. ANDREWS, & CATHLEEN BAKER
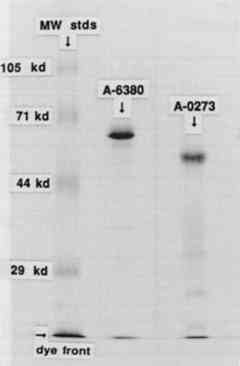
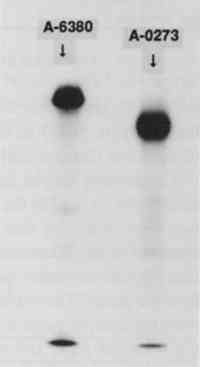
3 RESULTS AND DISCUSSION3.1 IODINATION OF α-AMYLASESThe SDS-PAGE gel of the iodinated amylases is shown in figure 2. This technique separates proteins on the basis of their molecular weight. The first lane, on the left, contains the molecular weight standards; the second lane contains the iodinated A6380 amylase; and the third lane contains the A0273 amylase. Both of the enzymes gave a major protein band upon staining. The less pure amylase contains fainter bands, indicating the presence of additional proteins in this preparation. According to the manufacturer, only about 25% of the less pure amylase is protein. An autoradiograph of the same gel can be seen in figure 3. The exposed dark bands on the autoradiograph indicate the presence of material that has been labeled by the radioactive iodine. Comparison of the stained gel with the autoradiograph shows that the
3.2 TREATMENT OF THE PAPER SAMPLES WITH IODINATED AMYLASESA total of eight experiments were conducted involving various rinsing procedures and various enzyme buffer solutions. Data from these experiments are compiled in tables 1–3. 3.2.1 α-Amylase A6380Table 1 contains data gathered on the more pure α-amylase, A6380. Initially the enzyme was used in a 10 mM sodium acetate buffer, pH 5. At the commencement of the experiment, we were not aware that 5 was not the optimum pH for A6380. This pH environment was selected because it was the pH recommended for α-amylase A0273 in conservation literature (Burgess and Charette 1981). The no-rinse samples show that 1–3% of the total cpm is retained in the paper samples. After two water rinses, less than 1% of the total cpm remained on the Whatman paper, whereas more than 2% of the total cpm remained on the Japanese paper. This was an unexpected result, since the Japanese paper is much less dense than the Whatman paper and was expected to rinse more thoroughly. Further water rinses did not remove appreciably more enzyme from either paper type. We reasoned that rinsing the paper first with the same buffer in which it was treated might help to keep the enzyme soluble during the A search of the biochemical literature on this amylase (Menzi et al. 1957; Ono et al. 1958) revealed its pH optimum to be 6 instead of 5, so we decided to try treating the paper with the enzyme at pH 6 in both a sodium acetate and a sodium phosphate buffer. Phosphate is a more effective buffer than acetate at this pH. The two buffers were tested at both 10 mM and 50 mM. A reduction in the amount of enzyme retained after rinsing was seen at this pH in both paper types. This reduction was particularly dramatic in the case of the Japanese paper, reducing the counts retained almost 20-fold. Use of either the 50 mM sodium phosphate or the 50 mM sodium acetate buffer at pH 6 resulted in the least amount of counts retained by either paper type. An additional experiment was performed with α-amylase A6380 to determine whether the binding of enzyme to the paper was due to the presence of substrate (starch) or an inherent property of the paper. The Whatman and Japanese papers, with and without a paste substrate, were treated with the enzyme in a 10 mM sodium acetate buffer, pH5. The results of this experiment are presented in table 2. The presence of the paste substrate on the papers did not significantly affect their retention of radioactivity, indicating that retention was an inherent property of the paper and not caused by the substrate. During this experiment the concentration of the enzyme was also varied to determine the effect of enzyme concentration on retention of 3.2.2 α-Amylase A0273The data obtained with the crude α-amylase A0273 are compiled in table 3. The published pH optimum is 5 (Matsubara et al. 1959), and all experiments were performed at this pH. The results for this enzyme confirm that four water rinses were no more efficient than two rinses in removing enzyme from the paper. The use of acetate buffer rinses followed by water rinses was again no more effective than rinsing with water alone. Again, adding a final ethanol rinse or using a suction rinse was no more effective in removing enzymes than water rinses alone. Raising the acetate concentration in the enzyme solution to 50 mM did not appreciably reduce the amount of enzyme bound to the Whatman paper. However, changing the buffer solution to 50 mM sodium phosphate reduced the amount of enzyme bound by 60%. It should be noted that with the crude enzyme approximately 75% of the solid weight of the preparation is not protein, at least according to the label on the bottle. Since the iodination labeled only the protein, the other 75% of the preparation is not labeled and therefore not detectable by these experiments. It was not necessarily a goal of these experiments to remove all of the starch substrate from the paper samples. However, when an iodine-potassium iodide reagent test for the presence of starch (Browning 1977) was performed on the paper samples following treatment, it was found for both enzymes that using the 50 mM phosphate buffer rather than acetate buffer with the enzyme was the most effective method for removing all the substrate within the given 45-minute period. |