RECENT SCIENTIFIC RESEARCH IN PAPER CONSERVATION
DIANNE VAN DER REYDEN
4 CONSERVATION TREATMENTS
The investigations reported in this section address changes to paper properties caused by various treatments. Conservators are cautioned to remember that the demands of good research limit the scope of experimental design, which cannot possibly be expected to incorporate all conditions of actual conservation treatment practice. The practicing conservator should recognize that reported data pertain to specific experimental conditions. However, the data may be correlated to actual practice by extrapolation based on an understanding of the interaction of paper properties. For instance, one might hypothesize that a bleach found to damage cellulose in a cotton paper may also damage cellulose in a paper made of more reactive ground wood pulp. By the same token, changes to properties caused by one solvent application technique may not occur with an alternate application technique. The conservator must also recognize that the interpretation of research data requires great care. Treatments cause changes to more than one property. Some property changes may be perceived as beneficial while others may be considered detrimental, and indeed, these initial assessments may be reversed over time as the object ages. Each treatment of an object must be considered individually to decide whether the net benefits outweigh the disadvantages, depending on the needs and character of that particular object. For example, washing might remove discoloration, but this benefit must be balanced against a loss or change in sizing. Sizing agents such as gelatin impart strength to paper (Barrett 1989). A loss of sizing might reduce breaking length by decreasing tensile strength. At the same time, a loss of sizing might improve fold endurance by decreasing stiffness. The changes in properties may or may not be relevant, depending on subsequent conservation treatment (i.e., resizing) or the needs of the object. For instance, an improvement in fold endurance might be more beneficial for paper used in a book than in flat artwork. Furthermore, experimental conditions must be carefully considered when comparing data from different projects. Minor variations, for instance in techniques of sample preparation involving wetting or drying, may inadvertantly affect properties of gloss or tensile strength. Modifications in degree of solvent saturation and direction of application, or in drying time, pressure, and technique, must be taken into account. All of these variables are factors that a conservator must consider when formulating treatment options, particularly regarding the most intrusive conservation treatments of all: those involving washing or deacidification, bleaching, solvents, enzymes, and sizing.
4.1 WASHING
Washing in paper conservation refers to overall treatment of paper with aqueous solutions, usually containing additives (such as wetting agents, solvents, or alkaline compounds) that serve to swell paper fibers and dislodge or dissolve impurities in order to aid in cleaning, neutralize acidity, or deposit an alkaline reserve to guard against future acid exposure (Daniels and Shashoua 1991). Early research found that washing with deionized or distilled water could cause a drop in pH and fold endurance, since such highly purified water can dissolve the stabilizing magnesium and calcium components of paper (Tang and Jones 1979; Nelson et al. 1982). On the other hand, alkalinization, either with low concentrations used for cleaning or higher concentrations used to deacidify paper, may cause color change, especially in papers containing lignin, the unstable impurity in ground or mechanical wood pulp (Burgess et al. 1990). In order to differentiat!#e papers that may be improved with selected washing techniques from those that may not, Burgess has analyzed naturally aged papers that were washed in deionized water and in water containing low (20 ppm) and high (200 ppm) concentrations of magnesium compounds, before and after humid thermal accelerated aging. Washing with deionized water did remove some beneficial calcium and magnesium salts. However, since washing also removed acid impurities, sample papers with high initial acidity that were washed actually maintained after aging a degree of polymerization greater than the unwashed untreated controls, which underwent more acid hydrolysis during aging. On the other hand, the degree of polymerization for papers with low initial acidity did indeed drop, relative to the controls, after washing with deionized water followed by accelerated aging. In addition, although some alkaline-treated, lignin-containing papers maintained greater mechanical strength with zero-span tensile measurements, an increase in concentration of some alkaline magnesium components produced an increase in yellowing of the lignin-containing papers. As a result, the authors concluded that, pending further studies on lignin-containing papers, institutions should “consider reducing the quantity of ground wood papers which are being treated by aqueous deacidification,” shifting priorities to materials with little or no lignin (Burgess et al. 1990).
Increase in yellowing may also occur in ligneous ground or mechanical wood pulp treated by solvent immersion (Lienardy and van Damme 1990). Ligneous paper washed with 30% isopropanol in tap water registered a drop in reflectance as compared to the control (fig. 6a). For all wood pulp paper samples, immersion for 5 minutes in alcohol (followed by washing in water for 30 minutes), caused an increase in fold endurance but a reduction in breaking length (fig. 6b and fig. 6c). Breaking length is the calculated upper limit of length of a uniform paper strip that would support its own weight if it were suspended at one end. It is a convenient measure for comparing the tensile breaking strength of papers of varying grammages (Caulfield and Gunderson 1988). A similar effect of alcohol was found in an earlier study, in which immersion of a mixed cottonwood pulp, alum rosin sized paper in 9:1 ethanol:deionized water appeared to cause a slight reduction in elongation to break (fig. 7a and fig. 7b) as compared to samples immersed in water alone (van der Reyden et al. 1988).
Fig. 7a.
Aqueous light bleached, 24 hours. Naturally aged Strathmore paper.
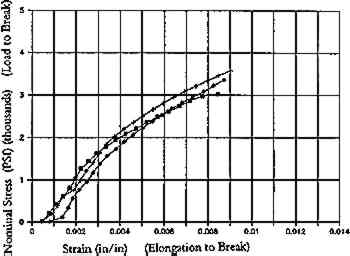 |
Fig. 7b.
Ethanol light bleached, 24 hours. Naturally aged Strathmore Paper.
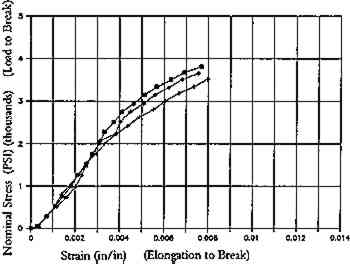 |
Reductions in breaking length, or in elongation to break, could result from a breakdown of sizing or the disruption of hydrogen bonding in paper immersed in organic solvent, which may in effect dehydrate or debond the paper. On the other hand, immersion in water swells fibers and releases the strain built into paper during the original drying of the sheet and subsequent aging. Aging stiffens paper as the random array of cellulose chains in the flexible amorphous regions of fibrils become more ordered and crystalline, held rigid by hydrogen bonds between the cellulose chains (fig. 2). Washing introduces water molecules that sever these bonds in the amorphous regions (fig. 3). Water can act as a plasticizer, swelling fibers and allowing fibrillar rearrangements that build in creep and encourage rebonding at less stressed sites upon drying. This phenomenon may account in part for the increase in stretch or elongation to break after washing (unsized) paper in alkaline solutions (fig. 4). Stretch is regarded by some in the paper industry as “one of the most important criteria for satisfactory behavior of paper in use” (Higgins and de Yong 1962). Furthermore, “a stress-strain curve in which the initial slope (Young's modulus) is high, where there is little curvature, and where the value for elongation to break is low, indicates a brittle material. A tough material is one that exhibits more viscoelastic behavior, a pronounced curvature, and a high elongation to brea” (Caulfield and Gunderson 1988).
4.2 BLEACHING
Bleaching, potentially damaging to paper and fibers, is nonetheless occasionally used by some paper conservators when washing fails to remove stains. Many paper conservators have become particularly interested in aqueous light bleaching, which consists of bleaching paper immersed in an alkaline solution by exposing it to light (Keyes 1980). Feller investigated the role of pH in aqueous light bleaching and found little difference in bleaching efficiency among solutions with pH 7–10, either before or after aging, on thermally discolored filter paper (fig. 8a) with little lignin or hemicellulose content (Lee et al. n.d.). He also found insignificant photochemical damage as manifested by color reversion or change in degree of polymerization after accelerated thermal aging of aqueously light bleached filter paper samples (fig. 8b). This parallels findings regarding mechanical properties of an earlier study (van der Reyden et al. 1988), which has been expanded to include accelerated aging (Schaeffer et al. n.d.). The tensile strength of cotton-fibered Whatman #1 paper aqueously light-bleached with UV filtration in an Atlas Weather-Ometer is statistically identical to all washed controls, with an increase in elongation remaining greater after aging than the aged untreated control (fig. 9a, fig. 9b). On the other hand, a naturally aged artists' paper with similar fiber composition,. but heavily sized with alum and gelatin underwent a reduction in stress to failure or load to break for samples immersed in a solution of calcium hydroxide (pH 9.5) in the Weather-Ometer (fig. 9c, fig. 9d). This situation is probably the result of a breakdown in sizing, especially when the paper is subjected to thermal degradation during aqueous light bleaching in the (warm) Weather-Ometer. The reduction in strain, or elongation to break, before aging might be attributable to thermal or photooxidation of the paper or sizing. However, after aging the strain measurements of both types of immersed samples are statistically identical to each other. Interestingly, the aqueously light-bleached samples underwent the least color reversion of all samples, particularly in yellowing, as measured by CieL∗a∗b∗ (fig. 9e). Lienardy and van Damme (1990) reported similar results for samples aqueously bleached by unfiltered sun and artificial light, although the samples underwent a drop in fold endurance, which may indicate ultraviolet degradation and/or a breakdown or alteration in the sizing of their naturally aged test papers.
Naturally aged test papers pose several problems with respect to good research design since such papers vary considerably in fiber, size, additives, and degradation products even within a single sheet. Such papers may also have low degrees of polymerization and tensile strength, which make changes difficult to measure. Consequently, naturally aged papers should be tested along with controls of similar fiber composition, which can be thermally pre-aged, and may provide the outer parameters for treatment evaluation. If relatively strong papers (such as the filter and artists' papers made by Whatman) are damaged by a treatment, naturally aged papers of lesser quality may be expected to be damaged as well.
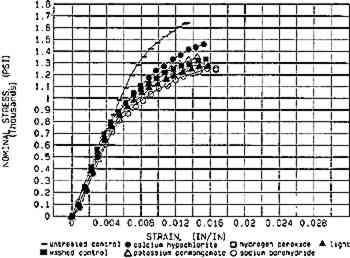
The effects of aqueous light bleaching have been compared to those of chemical bleaches (van der Reyden et al. 1988; Lienardy and van Damme 1989; Hofmann et al. 1990; Flieder et al. 1991). Findings before and after aging indicate that aqueous light bleaching, with UV filtration, causes no significant damage to the mechanical strength of a preaged cotton paper as compared to chemical bleaches (fig. 10) when all bleaching conditions are followed by extensive washing (Hofmann et al. 1992). Regarding chemical bleaches in general, Burgess (1990a) has shown that although both hydrogen peroxide and sodium borohydride produce excellent color stability with respect to reversion, increasing the pH of stabilized hydrogen peroxide to pH10 caused a decrease in the degree of polymerization by 21% in a 19th-century rag paper. However, sodium borohydride can actually stabilize damaged cellulose, reducing the color-producing double bonds of two types of carbonyls (aldehydes and ketones) back to original colorless alcohol species (Burgess 1988).
Fig. 10.
Comparison of aqueous light bleaching to some traditional chemical bleaches with respect to tensile properties (MD), both before (fig. 10a) and after (fig. 10b) aing. The sample paper was thermally pre-aged to simulate natural aging before treatment. (Source: Hofmann et al. 1992)
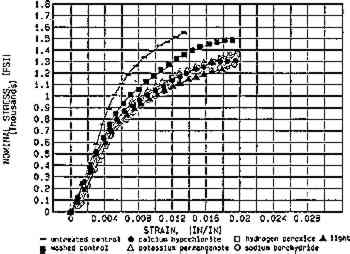 |
Fig. 10a.
Whatman 1 (pre-aged): Before accelerated aging
 |
Fig. 10b.
Whatman 1 (pre-aged): After accelerated aging
 |
4.3 SOLVENTS
Use of solvents can often preclude the need to bleach, by removing stains and adhesive residues, but paper conservators remain concerned about the effects of solvents on paper (MacKay and Smith n.d.). One problem, for instance, is that local treatments can sometimes produce tidelines which are only visible in ultraviolet light, or after aging of the treated object. In addition to the publications mentioned in the washing section, recent studies have focused on particularly solvent sensitive papers such as modern chemical watermarked papers (Watkins 1990), historic transp35arent paper (Flieder et al. 1988; Richardin et al. 1990), and coated papers (Baker et al. 1989). Such papers are affected by both choice of solvent agents and choice of application technique. Recent work has shown that choice of solvent diluent (such as ethanol, acetone, or toluene), affects properties of selected polymers (Hansen et al. 1991). A study comparing solvents and application techniques for stain removal (using immersion, suction table, and poultice) has documented alteration of surface fin46ish of paper, including break-up of impregnants, leading to a loss of translucency in tracing papers (fig. 11). The effects of solvents on coated papers parallel in some ways findings by paintings conservators on the reaction of pigment films to solvent swabbing and immersion (Hedley et al. 1990). For example, application of aqueous poultices to some coated papers may produce what appear to be tideline stains but are actually cracks (fig. 12). Attempts to reduce stains on coated papers with solvents could actually increase cracking (van der Reyden n.d.). Clearly, there is a need to continue to characterize specialty papers using Fourier transform infrared spectroscopy, gas chromatography/mass spectrography, and scanning electron microscopy/energy dispersive spectrometry in order to track effects of alternative solvent application techniques with SEM dot mapping and UV reflectance/transmission microscopy.
Fig. 11.
Scanning electron micrographs of a tracing paper (transparentized by impregnation with an acrylic resin)
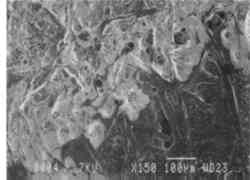 |
Fig. 11a.
Treated in the upper left section by immersion in ethanol, which caused a breakdown in the impregnant [150 x]
 |
Fig. 11b.
Detail, untreated area [3000x]
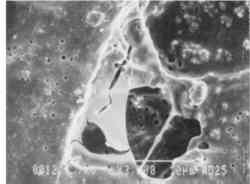 |
Fig. 11c.
Detail, treated area [3000x]
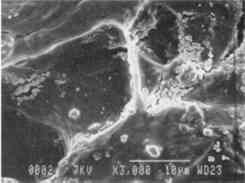 |
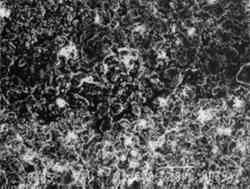
Fig. 12.
Scanning electron micrograph of a prepared ground paper (coated with barium sulfate and calcium carbonate in acrylic resin) treated with an aqueous poultice, which caused cracking along the perimeter of the poultice
 |
4.4 ENZYMES
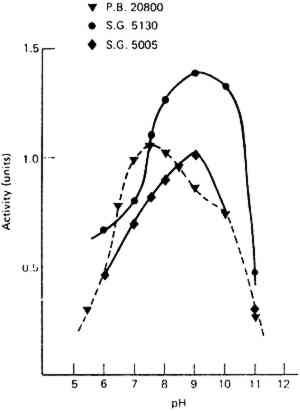
Like many other conservators, paper conservators employ enzymes to break down adhesives among other things. Burgess (1990b) has reported on criteria for enzyme selection and on the discrepancies between the working properties and information found in specifications on manufacturers' labels. Labels may record enzyme activity measured on substrates such as casein (which is different from adhesives generally encountered by paper conservators), as well as the pH at which the enzyme was assayed, rather than the pH for optimum activity (Grattan et al. 1987). In fact, some enzymes are biphasic, having high activity levels in both acid and basic pH ranges (fig. 13). Although enzyme activity approximately doubles in rate for each 10�C rise from 20–60�C, activity can be increased at lower temperatures by increasing the concentration of enzyme. However, another study, in which amylase enzymes were tagged with radioactive iodine isotopes, indicated that even though most of the enzymes were removed by rinsing the test papers twice with water, the amount of enzyme residue remaining in the paper after rinsing, although quite small, increased with an increase in concentration and purity (Andrews et al. 1990). The authors therefore recommended that conservators use the minimum amount of enzyme needed to do the job.
Fig. 13.
One example of biphasic enzyme activity of amylase P.B. 20800 with increased activity around pH 7 and 10. (Reprinted with permission from Grattan et al. 1987)
 |
4.5 SIZING
Paper conservators are concerned about whether any of the solutions discussed above alter or remove the sizing in paper, whether papers should be resized, and if so, with what (Barrett 1989). Barger has examined papers, from 17 disbound books, which after washing and deacidification were resized with gelatin, parchment, wheat starch, methylcellulose, and Klucel-G (Carapella et al. 1990). SEM examination revealed that the surface relief of the papers selected for treatment played a large role in the conformation and adhesion of sizing. For instance, gelatin adhered more poorly to high-surface-relief rag papers than to more planar wood pulp paper. The sizes tested did not appear to undergo significant morphological change after accelerated aging, aside from some curling and cracking of gelatin size. However, Feller's study of cellulose ethers found poor color stability, after accelerated aging, for hydroxypropyl cellulose powders like Klucel-G, with a 28% decrease in reflectance (Feller and Wilt 1990). Hydroxypropyl cellulose powders appear to be more susceptible to thermal degradation than other cellulose ethers because of a 60% degree of substitution of hydroxyls by etherifying reagents. Feller's study also found that in general, cellulose ethers with lower molecular weights tend to discolor more than those with higher molecular weights.
|









