CURRENT RESEARCH ON THE EFFECTS OF SOLVENTS AND GELLED AND AQUEOUS CLEANING SYSTEMS ON OIL PAINT FILMSJIA-SUN TSANG, & DAVID ERHARDT
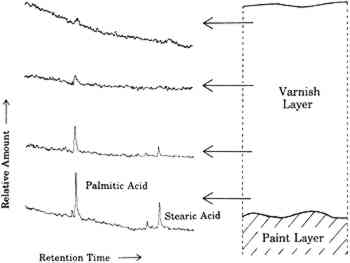
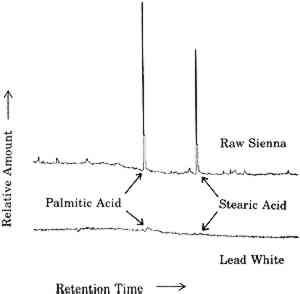
3 VARNISHES AS POULTICESPaintings are exposed to solvents at times other than during cleaning. One of the more prolonged exposures of a painting to solvents is during the application and drying of varnish solutions. Solvent evaporates from the surface of the varnish solution, but some also diffuses into the paint film. Solvent that diffuses into the paint film may dissolve soluble components. As this solvent eventually diffuses back into and through the varnish layer and evaporates from the surface, it may carry the soluble oil components with it. These involatile compounds may be left behind either in or on the varnish layer. This phenomenon is demonstrated by the following example. A layer of natural resin varnish was scraped from a small area of an oil painting in a series of ten steps. These ten scrapings were analyzed by gas chromatography according to previously reported methods to look for differences among samples from different levels in the varnish layer (Erhardt et al. 1988). Portions of the gas chomatograms where free fatty acids (palmitic and stearic) would appear are shown for four of these levels in figure 3. There are only traces of fatty acids in the top of the varnish layer. Larger amounts of fatty acids are seen in levels closer to the paint surface. A poulticing action of the applied varnish in moving these fatty acids from the paint layer to the varnish is not the only possible explanation, however. It is possible that fatty acids simply diffused into the paint layer over time or that fatty acids were present in the original varnish (which was present on the painting when acquired). To rule out these alternative explanations, a solution of Acryloid B72 was applied in xylene solution to five-year-old raw sienna and lead white oil paint films. Acryloid B72, manufactured by Rohm and Haas, Philadelphia, Pennsylvania, is a synthetic acrylic polymer commonly used as a varnish. It does not contain any fatty acids. After several days of drying, the B72 was peeled off and dissolved in methylene chloride. This solution was extracted with a solution of sodium bicarbonate, which was then separated, acidified, and extracted with fresh methylene chloride.
The fact that fatty acids are extracted from paint films by the application of varnishes is not necessarily cause for alarm. Any treatment of an oil paint film that involves the use of solvents will remove some material from the paint, even if in amounts detectable only by very sensitive analytical techniques. The presence of fatty acids in any amount does, however, demonstrate that an applied layer of varnish solution can act as a poultice, with the solvent drawing soluble materials from the paint film up into the layer of varnish. One does not apply just a varnish, one applies a varnish and a solvent. These results emphasize that the choice of solvents used in the application of a varnish to a painting is just as important as the choice of solvents used to clean the painting. Such considerations provide added importance to the efforts to find or develop varnishes that are and remain soluble in the mildest solvents possible (de la Rie and McGlinchey 1990). One important consideration of the poulticing effect of the application of solutions of varnish is in the interpretation of analytical results. Usually, the presence of fatty acids and terpenoid components in a single layer has been thought to indicate the use of an oil-resin mixture. Such mixtures, containing various proportions of oil and resin, have been used as glazes and varnishes. This work shows that fatty acids may be present in a resin layer due to extraction from the underlying paint layer rather than to the addition of oil to the resin. A modification of analytical techniques may be required to determine the source of the fatty acids. If a varnish is dissolved in an organic solvent and this solution is extracted with a weakly alkaline aqueous solution, any free fatty acids poulticed from the paint film would be extracted into the aqueous layer. If oil were a component of the varnish, there will be fatty acids present as glyceride esters. These esters will remain in the organic layer and could be detected by subsequent hydrolysis, methylation, and gas chromatographic analysis of fatty acids. |