CURRENT RESEARCH ON THE EFFECTS OF SOLVENTS AND GELLED AND AQUEOUS CLEANING SYSTEMS ON OIL PAINT FILMSJIA-SUN TSANG, & DAVID ERHARDT
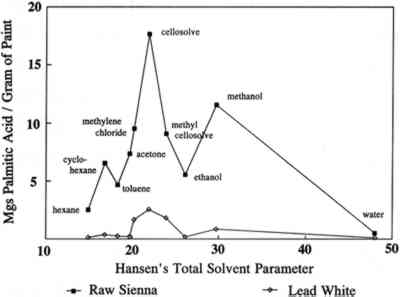
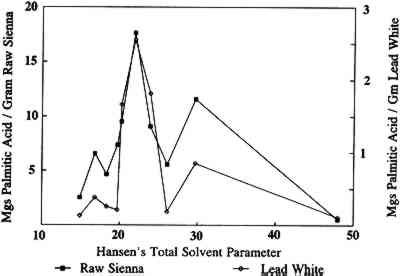
2 SOLVENTS AND SOLVENT PARAMETERSInitial studies carried out at the Conservation Analytical Laboratory, Smithsonian Institution, on the types and amounts of materials leached from oil paint films during soaking in various solvents have produced results that confirm Stolow's work and demonstrate the effects of pigments and age (Erhardt and Tsang 1990). Free fatty acids and their degradation products were found in the extracts, and the amount varied with the solvent, pigment, age of the film, and nature and length of solvent exposure. Materials other than oil components were also extracted from some samples. For example, wax and synthetic dyes were found in some extracts. Such additives often are found in paints, especially modern ones. Waxes can be used as extenders or may be added to pigments to aid in mechanical processing. Dyes may be used as extenders for inorganic pigments or may replace them entirely. The presence of such Lead white pigmented oil films were found in general to be less affected by solvents than films with other pigments that were studied (Erhardt and Tsang 1990). This is not surprising, since lead white speeds up the cross-linking of drying oils and may also form insoluble salts with the free fatty acids. Such pigment-medium bonding should reduce the sensitivity of the paint film to solvents. Raw sienna films, on the other hand, lost up to one-third of their weight after soaking in acetone and ethanol; most of this loss was due to the loss of pigment. This loss indicates a pigment-medium bond that is either weak or easily disrupted. We have looked at the amounts of material extracted from oil paint films by various solvents during varying types and lengths of exposure. The amounts of free palmitic acid extracted by various solvents over six hours versus the Hansen's total solvent parameter for each solvent are plotted in figure 1. Because of the limited size and number of aged samples available, only one measurement was conducted for each point. In a few cases where we have reported experiments, the results have varied within a range of about � 10%. The reliability of the data is further supported by the way in which trends in the data are maintained over time, through ranges of solvents, and for different types of samples. Hansen's parameters are measures of the contributions of three factors (dispersion forces, dipole interactions, and hydrogen bonding) to solvent interactions (Barton 1983). Hansen's total solvent parameter is a vector sum of these contributions and is a measure of the total solvent strength. Even though the amounts extracted from raw sienna films are consistently larger than those from lead white films, the shapes of the curves are very similar. This similarity can be seen more readily in figure 2, where the scale for the lead white plot is expanded. The similar shape of these two curves indicates that the lead white and raw sienna paint films differ only in the degree of their sensitivity to individual solvents, not in regard to which solvents they are sensitive. Thus, the effect of lead white pigments on paint films is to reduce the overall sensitivity of the film to solvents, not to shift this sensitivity to more or less polar solvents. This finding would indicate that the reduced sensitivity to solvents of lead white films is due to an increase in cross-linking, the formation of insoluble salts, or some other mechanism that has little effect on the polarity of the paint film. For instance, a large increase in oxidation of the paint film catalyzed
The sensitivity toward solvents of these paint films is not a smooth function of Hansen's total solvent parameter, however, as demonstrated by the two dips about the central peak. That this plot is not a smooth curve is the result of trying to plot sensitivity to solvents, which is a function of at least three distinct properties of the solvents, against a single parameter. Similar anomalies are encountered even in a standard Teas plot, which is an attempt to compress a plot of a function of three variables into two dimensions (Teas 1968). Michalski dealt with this problem by “decompressing” the Teas plot in order to make better sense of experimental results (Michalski 1990). Even clearer plots eventually may be obtained by abandoning the Teas plot format altogether and directly plotting results in three dimensions as a function of the three Hansen's parameters. |