CHEMICAL WATERMARKING OF PAPERSTEPHANIE WATKINS
ABSTRACT—An investigation of 20th-century Customark chemical watermarks beginning with methods of identification is reported. Experimentation with various conservation treatment steps on these materials gave conflicting results. Erasers seemed not to affect the watermark. Organic chemicals quickly altered the chemical watermark visually, yet under ultraviolet examination the mark appeared unaltered. Conversely, exposure to other solutions left the watermark visually undisturbed yet indistinct under ultraviolet illumination. It is hypothesized that component parts of the chemical watermark and perhaps paper brighteners, fillers, and/or sizing agents were moved by treatment methods, altering the mark. 1 INTRODUCTIONTHE TECHNOLOGY of chemically watermarking paper was first patented in 1959 by Frans V. E. Vaurio for the Customark Corporation, a subsidiary of the Fox River Paper Corporation (now Company) in Appleton, Wisconsin. The Customark Corporation remains the sole patent holder for chemical watermarking of paper, though it licenses the use of the technology to other mills. Since the development of the procedure, it has been used exclusively for the watermarking of stationery letter stock and envelopes. In 1988, approximately 6.5 million sheets (13,000 reams) of paper were chemically watermarked by the Fox River Paper Company alone (Carlson 1989). Paper conservators in the near future should expect to routinely treat papers with chemical watermarks in archival collections and perhaps in modern art pieces. “Watermarking” generally refers to a localized design, name, word, or date found in a sheet of paper. Strictly speaking, a watermark is a design. Smaller secondary names, words, and dates in the corners or opposite the main design are more accurately referred to as countermarks. However, the term “watermark” is used in this paper in the broader application, referring to the process of marking rather than to the content of the mark. The first watermarked Western paper dates from the 13th century. A cross and circle motif/design (Italy, 1282) is considered to be the first known Western example. Watermarks have not traditionally occurred in Eastern papers, although more contemporary examples exist. Watermarking originally might have been intended as an esthetic enhancement, a mark of quality, or a proprietary mark for the papermaker or the wealthy donor or client. Similar types of information, such as a particular brand of paper, manufacture from a specific mill, or depiction of a logo for a business or organization, are conveyed in watermarks today. Watermarks also relay historic information that can help date or geographically place a paper, thus validating a manuscript, document, or piece of art. The history and development of watermarks has been extensively researched. (See the bibliography for a brief listing of publications on the history, manufacture, and design of traditional watermarks.) Watermark designs are created by sewing metal wires or soldering metal stencils onto a wire screen. In making paper by hand, the wire, known as a mold, is dipped into the wet pulp slurry is in a tub known as a vat. When the mold is removed from the vat, the excess water drains by gravity from the slurry. The fibers are distributed over the mold surface and deposited between the raised wires. In making paper by machine, the stock is run over or under the dandy roll, a wire cylinder to which the design is attached and which displaces the wet fibers in the area of design. Designs may also be impressed by localized pressure between metal or rubber rollers or dies. However the mark is produced, the result is an area in the paper that is less dense and thus more translucent when held in front of a light source. (See Further Reading for additional explanations of the variations and nuances of traditional watermarking.) The high cost, inconvenience, and difficulty of producing intricate designs in conventionally manufactured wire or chiaroscuro1 watermarks were the impetus behind the invention of a simulated watermark in the commercial papermaking industry. The process of chemically watermarking paper allows for a greater diversity of applications at significantly lower cost. Yankoski (ca. early 1970s) cites the costs and limits a0111s being approximately $300 for a dandy roll with a minimum order of 200,000 papers (400 reams) versus $20 for a chemical watermark pattern and a minimum order of 12,000 papers (24 reams). With the Customark chemical watermark process, unmarked paper can be made in advance and stored. When an order is received, the watermark design is produced by stamping the pre-made papers and impregnating them with the patented compound under heat and pressure. While the manufacturer considers the resulting mark to be relatively inert (Yankoski ca. early 1970s). The marks in naturally aged samples are beginning to disappear into the surrounding paper structure. This phenomenon prompted interest in chemical watermarking and resulted in this investigation into some of the characteristics of modern chemical watermarks for the benefit of conservators. 2 SAMPLESCHEMICAL WATERMARKING is available under trade names such as Customark, Shadowmark, and Trustmark. Trustmark has a tagging material as a security feature that is mixed in the chemical watermark and changes color when exposed to chemical bleaches (Carlson 1988). Three chemically watermarked papers were supplied by the Fox River Paper Company for the purpose of experimentation. They were:
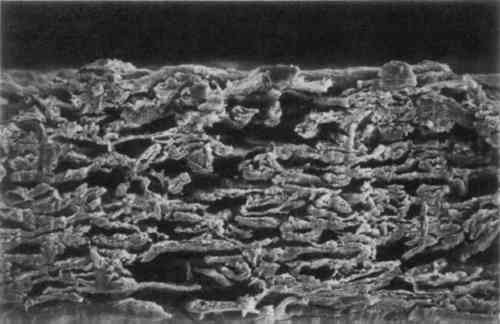
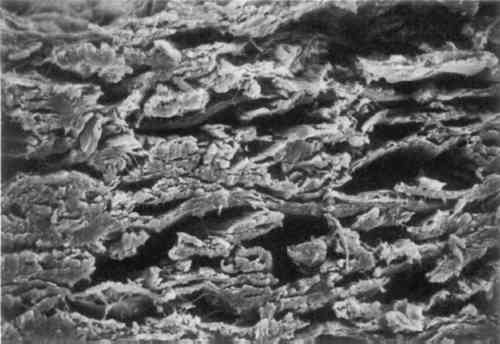
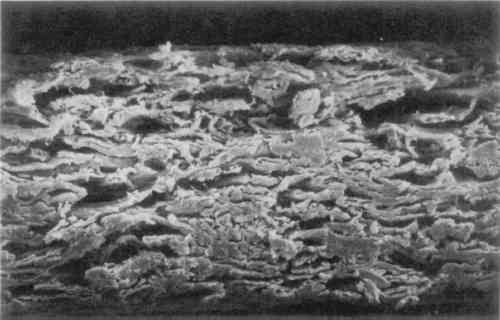
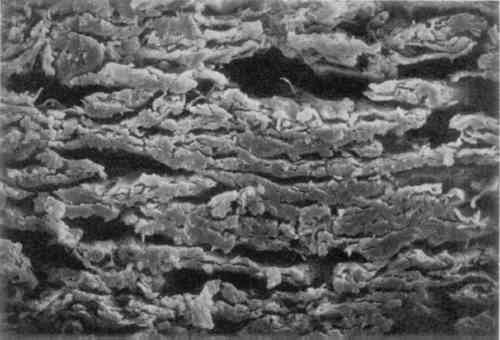
3 MICROSCOPIC EXAMINATIONSOME OF the objectives of an early chemical watermark patent were to form watermarks “involving the adequate displacement of entrained air,” “replacing air in a paper product with a material having an index of refraction similar to that of cellulose,” and “by impregnation thereof to replace entrapped air in a localized area” (Vaurio 1963). To determine whether the watermarked materials interact with the paper as the patent states, the papers were examined under magnification. Scanning electron microscope (SEM) images were taken of the cross-section of the GE watermarked paper. Two magnifications, 500� and 1000�, were used on both the plain paper and the chemically watermarked areas. The images produced are disappointing, as the material is indiscernible from the fibers. However, it is apparent that the chemicals “swell the fibers somewhat and close up the sheet” (figs. 1–4)(Block 1989).
4 NONDESTRUCTIVE IDENTIFICATION TECHNIQUESCHEMICAL WATERMARKS are indistinguishable from traditional watermarks to the unaided eye. Browning (1977) writes briefly of simulated or chemical watermarks and identification techniques. He cites beta radiography and short-wave ultraviolet illumination as methods that can distinguish between traditional and chemical watermarks. Short-wave ultraviolet radiation (180–280 nm) is the easiest method of identification. Chemical watermarks appear dark and dull because components of the watermark absorb the short-wave ultraviolet radiation rather than fluorescing with these wavelengths of light (fig. 5). It is important to inspect both sides of a paper for a chemical watermark, as the marks are better seen on the side of impregnation.
Stationery papers often contain bluing agents or optical brighteners, which fluoresce brilliantly, offering a contrast to the chemical watermark and aiding in the identification process. Chemical watermarks on unbrightened papers lack this contrast and can be harder to see. Fortunately, these marks can be distinguished by using illumination from long-wave ultraviolet sources (300–400nm). Under long-wave ultraviolet illumination, the marks on unbrightened papers appear lighter than their surroundings; they fluoresce as an opaque white in contrast to a dark, nonfluorescing background. Since the traditional watermark is made of the same material as the rest of the paper, it will react like the paper to ultraviolet light, whether or not the paper has been optically brightened. Short-wave ultraviolet radiation is more damaging to the human eye than the long-wave ultraviolet radiation commonly used by conservators. Protective glasses should always be worn when using short-wave ultaviolet radiation, and one should never look directly at the light source. Grenz rays at 7kV were used to radiograph two traditionally and two chemically watermarked papers.2 The traditional watermarks and the paper formation were faithfully recorded on film. Only the paper formation is noticeable on the film exposed under the chemically watermarked papers. This phenomenon occurs because the traditional watermark is a physical manipulation of the paper, whereas the chemical watermark acts more like a size in the paper. Since low-energy X-radiography will not detect organic resins, the chemical watermark is not recorded on film. Rantanen (1989) suggests that an identification can be made under the microscope using raking reflectance illumination. The indented or embossed nature of the traditional watermark will produce shadows. In contrast, variations in surface gloss will sometimes reveal the chemical watermarks as sitting on the surface of the paper. While the differences are easily observed on newer papers, an aged artifact may have been flattened through use, repair, or storage, making positive identification difficult. Placing a blue filter over the light source enhances the effectiveness of this technique. With the filter, the chemical watermarks appear dark on an even surface, while traditional watermarks appear the same color on an uneven surface. The sensitivity of the observer's eye and familiarity with these subtle characteristics is essential when relying on visual microscopic identification techniques. 5 CHEMICAL ANALYSIS OF THE MATERIALTHE TECHNOLOGY of chemically watermarking paper is considered a trade secret because it is owned and leased solely by the Customark Corporation. When patenting a new process or discovery, companies often protect themselves by listing multiple formulas and a variety of possible materials in a range of different combinations. Therefore, it is impossible to determine with certainty directly from the patents the components and proportions necessary for the chemical watermarking process. An attempt to determine the chemical content of the watermarks in the three samples was made by performing analytical tests. The United States patents (Vaurio 1963, 1964; Skofronick and Vaurio 1966; Skofronick 1969) cite hexamethoxy methylmelamine, melamine formaldehyde, diethyleneglycol monomethyl ether (Carbitol), and epoxy as the possible primary components of a chemical watermark. Based on the repetitions of The test for the detection of formaldehyde yielded a positive indication of acetaldehyde in all three samples. Other tests to identify the chemical components of the watermark were negative. However, the results do not necessarily mean that the materials for which tests were chosen are not used in chemical watermarks. Different samples or more sensitive test methods might reveal more information about the resins used. In an effort to obtain information about any possible surface treatments or paper components that might affect the results of later experimentation, standard tests for groundwood pulp, starch, gelatin, and alum were also run (Browning 1977).4 The papers did not contain lignin as indicated by tests with phloroglucinol. The tests for alum and starch were positive, indicating the presence of both. The test for gelatin did not indicate the presence of proteins. 6 SUMMARY OF EXPERIMENTAL PROCEDURESCHEMICALLY WATERMARKED stationery is most apt to appear in collections of archival and historic interest. In both instances, large quantities of documents are collected. Storage and problems requiring conservation attention are often considered en masse. Types of damage typical of these artifacts include soiling, stains from fastening implements (paper clips, rubber bands, and staples), tears, residue from pressure-sensitive tapes and adhesives, water and mold damage, embrittlement, and overall discoloration. Erasers, aqueous alkaline solutions, and organic solvents are routinely used during the treatment of paper artifacts. Therefore, the interactions of the following materials with chemical watermarks were studied: seven types of erasers; four aqueous alkaline solutions; eighteen organic solvents; and seven bleaches and bleach neutralizers (antichlors). Untreated papers were subjected to humid and dry artificial aging in an effort to replicate the condition of naturally aged sheets. All three chemically watermarked sample papers were used for every test. Observations under normal, normal transmitted, and short-wave ultraviolet illumination were made. The results from these experiments were recorded through written and photographic documentation. 7 EXPERIMENTAL PROCEDURES7.1 SURFACE CLEANINGERASERS WERE tested to determine whether oil, plastic, or rubber components traditionally present had any adverse effects on the chemical watermark. The erasers chosen were Artgum, Kneaded, Magic Rub, Opaline, Pink Pearl, Staedtler Mars Plastic, and Groomstick. The watermarked area of the paper was erased 25 times in a small circular motion characteristic of traditional cleaning methods. Any changes were noted. The crumbs from the erasure procedure were gathered and laid on the watermarked area. A thin polyester film was placed over the crumbs to reduce displacement possibilities. The papers were then stored on top of a blotter in a metal flat-file drawer away from light. After eight weeks, the papers were examined, and changes were recorded. 7.1.1 ResultsThe plasticizers, oils, or rubber components in the erasers used for this test did not appear to alter the watermarks. With the exception of Groomstick, the erasers did not seem to damage the paper. As viewed under normal illumination after eight weeks, all three papers darkened in the area in contact with the Groomstick. The darkened area in the JCC sample fluoresced a dull, opaque white under both short- and long-wave ultraviolet illumination. 7.2 EXPOSURE TO SOLVENTSA variety of organic solvents is used to remove stains and adhesives from paper. To determine the parameters of susceptibility to damage from such applications, the following solvents were chosen for testing: acetone, amyl acetate, carbon tetrachloride, diacetone alcohol, dichloromethane, dimethylformamide, ethanol, methanol, methoxy magnesium methyl carbonate in methanol and trichlorotrifluoroethane, methyl ethyl ketone, naphtha, petroleum benzine, 2-propanol, tetrahydrofuran, trichloroethylene, toluene, and xylenes. Acetic acid (2% in deionized water, pH 5.5) was also used. Three watermarked areas of each sheet were tested as follows: one drop in each area, allowed to evaporate thoroughly, followed by another drop in two of the areas, thoroughly dried, and ending with a third drop in one of the areas. A nonwatermarked area was similarly exposed to each solvent for comparison. Testing was completed within the fume extraction hood, and observations were noted. 7.2.1 ResultsThe chemical watermarks were moved or eliminated with the application of every solvent chosen. A ring of what appears to be watermark material was found encircling the test spots in all papers after the solvents evaporated. Under short-wave ultraviolet illumination, the watermarks appear unaltered, with well-defined edges. The exceptions were the watermarks tested with acetic acid and dimethylformamide. The application of acetic acid created lighter white spots; the application of dimethylformamide created dark bluish dots encircled by a very bright fluorescent ring formed by the movement of the optical brightener in the paper. 7.3 EXPOSURE TO AQUEOUS ALKALINE SYSTEMSThe water-based solutions sat on the surface of the papers, making the dropper technique an inefficient test method. Therefore the papers were immersed in trays of the aqueous alkaline solutions. The solutions used for testing were deionized water (pH 7.0) ammonium hydroxide in deionized water (pH 8.0–9.0); calcium hydroxide in deionized water (pH 8.0–9.0) and a saturated solution of calcium hydroxide in deionized water (pH 12.0). For each solution samples of all three papers were immersed in the same bath, and polyester film was placed on the water surface to ensure immersion of the papers and to prevent evaporation of the liquid. One sheet of each sample was removed after 15 minutes; the remaining three papers were removed after 2 hours. Upon removal, the samples were left to air dry on a polyester screen. After drying, they were compared to a control piece in normal and 7.3.1 ResultsTraditional watermarks become more translucent when wet. The chemical watermarks also reflected the color of the underlying trays to an unusual degree. While drying, the watermarks appeared to be opaque and diffusing into the paper. Once completely dry, the watermarks again became translucent and the papers returned to being opaque. After drying, there was some blurring of the watermarks in most of the papers. This loss of sharpness seemed to increase with longer immersion times. It was particularly apparent in the GE shadowmark sample and least apparent in the nonbrightened JCC sample. Under short-wave ultraviolet illumination, the watermarks appeared faint and indistinct from the paper. A fluorescing component or components from the watermark or paper may have been solubilized and redeposited across the surface, shielding the watermark from the ultraviolet source. 7.4 EXPOSURE TO BLEACHES AND BLEACH NEUTRALIZERSSeven chemicals used for bleaching and bleach neutralizing (antichlors) were tested on the watermarks and the surrounding paper areas: chloramine-T (sodium toluene-4-sulphon-chloroamide), chlorine dioxide, hydrogen peroxide, sodium borohydride, sodium hypochlorite, sodium meta-bisulfite, and sodium thiosulfate. A 5% solution of each chemical in water was prepared for testing. (The exception was chlorine dioxide, which was prepared at 2%.) Solutions at a concentration of 5% are higher than those normally used in conservation practice and were chosen as extreme conditions under which to test the watermark material. The concentrations were kept the same so that the effects could be compared more accurately. Most of the testing was done in a fume extraction hood. In each case, the chemicals were poured onto the papers in the area of the watermark and allowed to sit for 15 minutes. The excess was drained off, and the papers were blotted, then allowed to air dry on a polyester screen. Afterward, the papers were immersed in a deionized water bath for one-half hour and again allowed to air dry. The papers were observed with normal, normal transmitted, and long- and short-wave ultraviolet illumination. The effects of the chemicals on the watermarked designs were recorded during the procedure, after the bleaching, and after the final rinsing. 7.4.1 ResultsTesting produced inconsistent and contradictory results. The type of watermark, whether line or shadow, seemed to determine whether a bleach or bleach neutralizer was detrimental. Further testing with various concentrations and complete bleaching procedures might help obtain more applicable information. Chloramine-T diminished all three watermarks. This effect was noticed visually with normal transmitted light and under long- and short-wave ultraviolet illumination. Sodium thiosulfate and sodium meta-bisulfite either blurred or obliterated the BG and GE watermarks; the JCC paper had a mottled fluorescence across the surface, but the watermark remained sharp. Hydrogen peroxide left the BG watermark fainter under all types of illumination but did not seem to damage the GE and JCC watermarks. Watermarks treated with sodium borohydride appeared very faint but retained sharpness of definition under all illumination methods. Sodium borohydride also caused the papers to blister. Chlorine dioxide and sodium hypochlorite did not appear to alter any of the watermarks. Watermarks tested with both of these bleaches appeared dark on the obverse and fluoresced on the reverse under short-wave ultraviolet illumination. The appearance of the watermark under ultraviolet illumination before testing was dark on the reverse and unseen or white on the obverse. All the chemicals except hydrogen peroxide and sodium thiosulfate eliminated the cream colorant in the JCC paper. 7.5 ARTIFICIAL AGINGNaturally aged, chemical watermarks in the collection of the Dard Hunter Paper Museum at the Institute of Paper Chemistry (now the Institute of Paper Science and Technology, Inc.), seem to be diffusing into the paper support and disappearing. Yet when viewed under short-wave ultraviolet radiation, the designs look crisp and precise. Natural aging, cross-linking of the material, or uncontrolled fluctuating environmental conditions in the museum may be the cause of this phenomenon. In an attempt to simulate the diffusion of the watermarks, the papers were subjected to two types of artificial aging. Three samples (one sample sheet of each paper) were placed in the RH-controlled environmental aging chamber (Blue M Electric Humid-Flow Combination Temperature and Humidity Chamber) at 88�C (� 0.5�C) (190�F [� 1�F]) at 55%–60% RH for six days. Three more samples were placed in a dry oven (Lab-Line Imperial II Radiant Heat Oven) without exposure to humidity at 100�C (212�F) for six days. The papers placed in the humid aging oven darkened and turned brown. The watermarks were darker than the paper and opaque. All translucency of the watermark was lost. The samples placed in the dry oven were slightly brown and, similar to the museum's specimens, had diffuse, translucent watermarks that were still sharp under short-wave illumination. 8 SUMMARY OF EXPERIMENTAL RESULTSCHEMICAL WATERMARKS are complex structures that defy simple interpretation. Their behavior is further complicated by the paper on which they are placed. However, some preliminary conclusions can be drawn. Erasers do not appear to damage the watermarks. Organic solvents may be dissolving various components of the chemical watermark, altering the refractive index between the paper and the watermarked area. The solvent-treated watermarks appear unaffected when viewed under ultraviolet illumination, suggesting that the particular components responsible for the ultraviolet absorption of the watermarks are not being moved. Paper additives such as dyes, intensifiers, brighteners, sizing agents, and clays, minerals, and other fillers may also dissolve in various organic solvents, especially when present in combination with the chemical watermarks. It appears that the areas of chemical watermarks should not be treated with organic solvents. Even the laid and chain lines of a paper with a chemical watermark should be carefully examined and tested before any treatment, as it is possible such lines may also be chemically produced (Carlson 1990).5 Neutral aqueous, alkaline, and bleaching solutions dramatically change the visual and fluorescent properties of the watermarks. The chemical watermarks diffused into the papers when placed in aqueous and alkaline solutions and air dried, much like the effect seen with paper chromatography. Under ultraviolet illumination the watermarks on the side of impregnation gain an opaque white fluorescence, compared with the dark absorption seen prior to immersion. After immersion the chemical watermarks on the side opposite impregnation appeared blurred, dull, and dark under ultraviolet sources. Bleaches and antichlors seem to alter the paper additives (such as fluorescent agents, sizes, and dyes) and the chemical watermarks. The chemical watermarks and some papers seemed to lose intensity and appeared significantly changed when viewed under short-wave ultraviolet illumination. Many marks exhibited a white opaque fluorescence on the side of impregnation, and a dull absorption on the side opposite impregnation as seen in the water-immersed samples. These findings conflict with statements from Browning (1977) and Yankoski (n.d.). Browning claimed chemical watermarks to be “a polymerizable organic material which is not easily removed by solvents.” Yankoski cited a chemical watermark as a “translucent mark that is indelible, tamper proof and permanent.” However, Carlson (1988) stated that a chemical watermark is most definitely not indelible or tamperproof and that organic solvents would probably disrupt the marks. Artificially aging the samples furthered understanding of the material. Humid oven aging turned the watermarks an opaque brown that was darker than the surrounding paper and diminished the translucency of the mark. The watermarks aged under dry-heat conditions diffused into the paper, yet they remained translucent. The paper also darkened significantly. The artificially aged marks appeared sharper under ultraviolet illumination than under normal illumination. In the humid oven-aged samples, moisture or moisture in combination with heat may have cured or cross-linked a component of the watermark mixture, thus changing the color and refractive index properties of the mark. A possible explanation for the loss of clarity in the dry oven-aged watermark sample is that the lower-weight molecular components were being volatilized or melted into the surrounding paper structure. 9 CONCLUSIONS9.1 IDENTIFICATIONTHE NONDESTRUCTIVE identification methods that distinguish between traditional and chemical watermarks are:
9.2 TREATMENT EFFECTS
Experimentation often produces more questions than answers. This project is no exception, and the results of the tests are still being considered. Additional in-depth experiments are needed to better understand the characteristics and conservation treatment limitations of chemical watermarks. ACKNOWLEDGEMENTSGEORGE BOECK, formerly of the Dard Hunter Paper Museum at the Institute of Paper Chemistry in Appleton, Wisconsin (now the Institute of Paper Science and Technology, Inc., Atlanta, Georgia), brought the watermarks to my attention during the summer of 1988. Ken Carlson of the Fox River Paper Company, Appleton, Wisconsin, generously donated the sample papers and courteously answered questions concerning this technology. I am grateful to Walter J. Rantanen, fiber specialist at the Institute of Paper Science and Technology, Inc., for sharing his expertise and enthusiasm about this material. The SEM analysis performed by Mary Block, fiber specialist at the Institute of Paper Science This project was partially supported by a spring 1989 student award from the Office of the Dean of the Faculty of Arts and Humanities, State University of New York College at Buffalo. The work was completed as partial fulfillment of a master's degree in art conservation. The samples and documentation are on file at the Art Conservation Department, State University College of New York at Buffalo, New York. NOTES1. These watermarks are also referred to as “Shadecraft” (American Paper and Pulp Association), “Shaded” (Plank Dandy Roll Company), “Shadow” (Fox River Paper Company), and “Light-and-shade” (Dard Hunter). 2. Both beta and Grenz radiography will record low-density materials, such as paper, on film. During this research, Grenz radiography was used because of the availability of equipment. The use of Grenz radiography also allows for shorter exposure times, larger film sizes, and sharper film images than does beta radiography. 3. The determination of epoxy resins through the detection of nitrogen, p. 250; the determination of formaldehyde using phloroglucinol, p. 254; the determination of urea- or melamine-formaldehyde using fuschsin hypochloride, p. 255; and the determination of melamine using infrared spectrometry, p. 263. 4. The determination of groundwood using phloroglucinol (TAPPI #T-401), p. 73; the determination of the presence of starch using the iodine test, p. 93; the determination of the presence of gelatin using the Biuret test, p. 103; the determination of the presence of alum using the aluminon test, p. 217. 5. Chemically applying laid and chain lines has been done in the past. However, clients tend to prefer the texture of sheets with traditionally produced laid and chain lines. Therefore, chemically produced laid and chain lines are not very common. REFERENCESBlock, M.1989. Letter to author concerning Institute File No. 89-70204, Institute of Paper Chemistry, Appleton, Wis. March 22. Browning, B. L.1977. Analysis of paper. 2d ed.New York: Marcel Dekker. Carlson, K.1988. Personal communication, November 23. Carlson, K.1989. Personal communication, May 9. Carlson, K.1990. Personal communication, April 13. Rantanen, W.1989. Personal communication, April 7.
Skofronick, B. D.1969. Method of rendering shadowmark opaque by solvent treatment. U.S. Patent #3,443,979 (continuation of series #536,487). Assigned to Customark Corporation, Appleton, Wis. Skofronick, B. D., Water-treated shadowmarks. U.S. Patent #3,486,923 (continuation of series #525,343). Assigned to Customark Corporation, Appleton, Wis. Skofronick, B. D., and F.V.E.Vaurio. 1966. Chemical watermark applied on finished paper. U.S. Patent #3,293,062. Assigned to Customark Corporation, Appleton, Wisconsin. Vaurio, F.V.E.1963. Paper product with watermark and process therefor. U.S. Patent #3,085,898. Assigned to Customark Corporation, Appleton, Wis. Vaurio, F.V.E.1964. Paper product with chemical watermark and means for making same. U.S. Patent #3,140,959. Assigned to Customark Corporation, Appleton, Wis. Yankoski, J. J. n.d. [ca. mid-1960s–early 1970's]. Watermarks … are they practical? In Focus (Appleton, Wis., Fox River Paper Corporation) 2. FURTHER READINGCHEMICAL WATERMARKSDidwania, H. P.1966. Revolution in the process of watermarking. Indian Print Paper (England) 32(2):27–30. Roberts, E., Jr.1964. There's no mystery about watermarks (knowing something about them can be useful). American Pressman74(3):6, 8, 10. Weiner, J., and K.Mirkes. 1972. Watermarking. Bibliographic Series, no. 257. Appleton, Wis. Institute of Paper Chemistry. TRADITIONAL WATERMARKSAPPA. 1965. Watermark. In Dictionary of paper. New York: American Paper and Pulp Association. Briquet, C. M.1923. Dictionaire historique des marques du papier, vols.1–4. 2d ed.Leipzig: Karl W. Hiersemann. Gravell, T. L.1979. A catalogue of American watermarks, 1690–1835. New York: Garland Publishing. Gravell, T. L.1983. A catalog of foreign watermarks found on paper used in America, 1700–1835. New York: Garland Publishing. Heawood, E.1950. Historical review of watermarks. Amsterdam: N. V. Swets and Zeitlinger. Heller, J.1978. Papermaking. New York: Watson-Guptill Publications. Hunter, D.1916. Handmade paper and its watermarks: A bibliography. New York: The Mill, Marlborough-on-Hudson. Hunter, D.1978. Papermaking: The history and technique of an ancient craft. 1943. New York: Dover Books. Le Barre, E. J.1937. A dictionary of paper and papermaking terms. Amsterdam: N. V. Swets and Zeitlinger.
Leif, I. P.1978. An international sourcebook of paper history. Hamden, Conn. Archon Books, Shoestring Press. Library of Congress. 1968. Papermaking: Art and craft.Washington, D.C.: Library of Congress. Young, L. C.1973. Materials in printing processes. New York: Visual Communication Books, Hastings House Publishers. SOURCES OF MATERIALS USEDOpaline dry cleaning pad, Patent #2, 287, 477Durasol Chemical Co., Boston, Mass. Kodak Wratten deep blue gelatin filter #47, 75 � 75mm (3 � 3 in), catalog number 149 5787Eastman Kodak Co., Rochester, N.Y. Kneaded Eraser #1225, Pink Pearl #101 eraserEberhard Faber, Inc., Wilkes-Barre, Pa. Artgum #211 eraser, Magic Rub #1954 eraserFaber Castell, Lewisburg, Tenn. Staedtler Mars Plastic eraser #526–52Staedtler Mars, Nuremberg, West Germany Groomstick natural rubber, molecular trap eraserTalas (distributor), New York, N.Y. Methoxy magnesium methyl carbonate, in menthanol and trichlorotrifluoroethane, (Wei T'o Solution No. 2)Wei T'o Associates, Matteson, Ill. AUTHOR INFORMATIONSTEPHANIE WATKINS is a Getty fellow in paper conservation at the Northeast Document Conservation Center in Andover, Massachusetts. She previously worked at the Intermuseum Laboratory in Oberlin, Ohio and interned at the Library of Congress, Phillips Collection, International Museum of Photography at George Eastman House, and Dard Hunter Paper Museum. She has received a master's degree in conservation from the State University College at Buffalo, and a bachelor's degree in fine art from Virginia Commonwealth University and had a Guggenheim student fellowship. Her interest in modern processes and materials is stimulated by her background in the fine arts. Address: Northeast Document Conservation Center, 100 Brickstone Square, Andover, Massachusetts 01810.
 Section Index Section Index |