DARKENING OF PAPER FOLLOWING EXPOSURE TO VISIBLE AND NEAR-ULTRAVIOLET RADIATIONS. B. Lee, J. Bogaard, & R. L. Feller
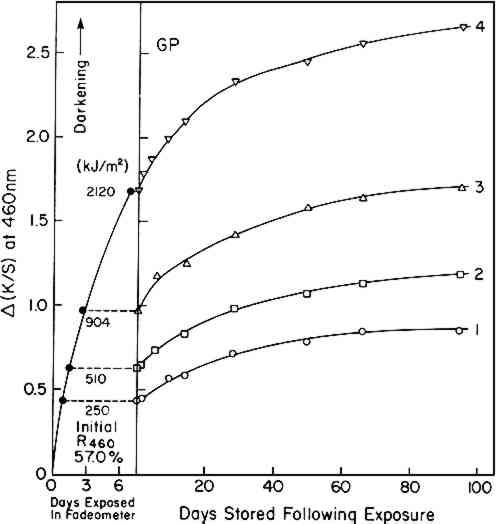
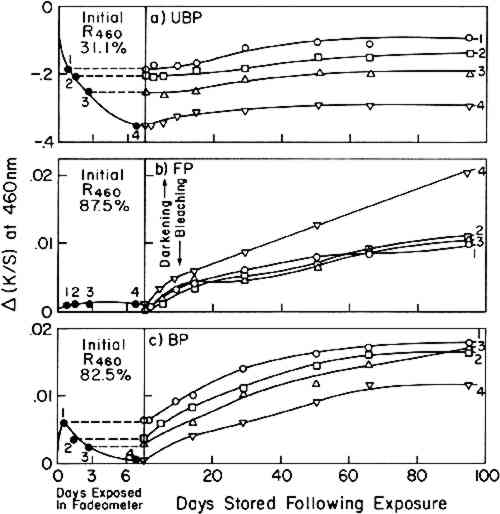
ABSTRACT—In a series of experiments using three different sources of visible and ultraviolet radiation, post-irradiation darkening at room temperature of water-leaf papers made from various pulps is shown to be chiefly influenced by the content of lignin and by the pH of the test sheets. 1 INTRODUCTIONFOR MANY YEARS, it has been known that exposure of paper to very short-wavelength ultraviolet, such as 254 nm radiation, will induce post-irradiation effects, the specific results of which are influenced by both internal and external factors.1–4 External factors during irradiation and subsequent storage include wavelength of irradiation, temperature, humidity, and atmospheric conditions. Internal factors relate to chemical composition of the paper: acidity, carbonyl groups, and the content of hemicellulose, extractives, and lignin. It is also known that exposure to visible and ultraviolet radiation results in complex discoloration effects both during and after exposure.5 Nonetheless, the fact remains that few studies have been carried out specifically to elucidate the behavior of papers subsequent to exposure to the wavelengths of visible and near-ultraviolet radiation normally involved in the display of works on paper, approximately 320 to 760 nm. Launer and Wilson found that soda-sulfite paper irradiated under conditions that resulted in bleaching (330-440 nm radiation; sheet temperature 30�C) yellowed during a subsequent storage period of 15 months.6 MacMillan reported the sensitivity to thermally-induced aging following exposure of test papers in the Fade-ometer� to be a function of the aldehyde-group content in the cellulose molecules.7 This particular observation suggests that the observed post-irradiation darkening under visible and near-ultraviolet radiation may essentially be the result of thermal-aging processes even though the level of temperature of the paper when stored away from the light may not be far from normal room conditions. This view was expressed by Launer and Wilson. One may reasonably regard the widely reported post-irradiation effects that occur as a result of exposure to short-wavelength radiation (below 280 nm) to have only minor relevance to museum and library problems. It is far more important for conservators to increase their knowledge of the effects of exposure to wavelengths of near ultraviolet and visible radiation. Reported herewith are data on the effects of different (a) exposure dosages, (b) initial pH values in the sheet, and (c) levels of hot-alkali-soluble matter, gamma cellulose and lignin upon the discoloration that occurs during The primary objectives of the studies reported here are to determine (1) whether paper exposed to these wavelengths will undergo post-irradiation darkening under rather ordinary conditions of storage away from the light, (2) whether the pH of the paper has an influence on post-irradiation darkening under these circumstances, and (3) whether the content of lignin, rather than hot-alkali-soluble (HAS) matter, has a marked influence on the phenomenon. The answer to all three questions is affirmative. The information is of value with respect to the behavior that may be expected as the result of the display of works on paper. The results are not intended to be informative with respect to the practice of intentionally bleaching by light while discolored papers are immersed in an aqueous bath. That will be the subject of a publication to appear elsewhere. 2 RESULTS AND DISCUSSION2.1 Effect of Exposure TimeESPECIALLY PREPARED WATER-LEAF (without size) test sheets based on four types of paper stock, designated GP (groundwood pulp), UBP (unbleached pulp), FP (filter paper) and BP (bleached pulp) (see Table I regarding symbols and description of the pulps) were exposed during an initial series of tests for 18.8, 37.5, 65.8, and 155 hours in the Atlas Electric Devices xenon-arc Fade-ometer� equipped with Pyrex� filters. The respective net exposed energy, monitored at 420 nm, was 250, 510, 904, and 2120 kJ/m2. Reflectance readings at 460 nm, taken from measurements of the spectrophotometric curves, were noted at various times on the samples during exposure in the Fade-ometer� and during a subsequent period of storage in the dark in a steel cabinet in a constant-temperature and constant-humidity room maintained at 23�C and 50% RH. Darkening or bleaching is expressed in terms of the Kubelka-Munk function for the ratio of absorption coefficient, K, to the scattering coefficient, S, where K/S = (1 − R)2/2R, and R is the decimal fraction reflectance at 460 nm of a stack of six sheets of the paper being tested, relative to a standard white of barium sulfate. Table II compares K/S values with percent reflectance; increased K/S indicates darkening. TABLE I Characteristic of Paper Samples Used (Before Exposure to Light) TABLE II Comparison of Reflectance Values with Kubelka-Munk K/S Function∗ Changes in the K/S values, Δ(K/S), are plotted against exposure and storage time in Figure 1 for the GP test sheets and in Figure 2 for the UBP, FP, and BP sheets. [The more commonly used expression, post-color number,8 would be 100 times this, based on measurements with a narrow-band-pass filter peaked at 457 nm. However, with the introduction of modern spectrophotometers, it has proved convenient for this laboratory to use the reflectance at 460 nm. The net change is practically the same whether the reflectance at a wavelength of 457 or 460 nm is used.]9
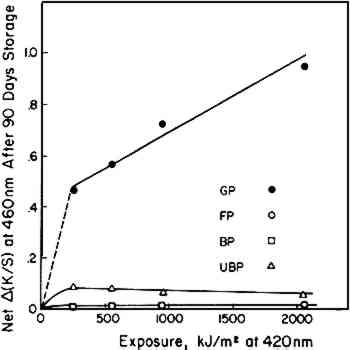
Three papers employed in these experiments were low in lignin. These did not change in color as much as the test sheets made with the groundwood pulp (GP). This can be seen in the much smaller changes in K/S values, Δ(K/S), in Figure 2 as compared to Figure 1. Subsequent to exposure, the filter paper (FP) darkened least of all, as one might expect in the case of a high-alpha-cellulose pulp. The particular sample of unbleached pulp (UBP) used in these tests was rather dark colored in its initial state, perhaps caused by alkalinity in the pulping treatments. In a number of laboratory experiences with this pulp we have found that it tends to bleach, at least Regardless of whether exposure in the Fade-ometer� had caused bleaching or darkening, all of the exposed samples subsequently darkened when stored in darkness in a laboratory cabinet at 50% RH and 23�C (which we shall designate as post-irradiation darkening).10 In our experience we have observed that test sheets containing significant amounts of lignin tend to darken when exposed to near-ultraviolet radiation and higher-than-normal temperatures. Thus, one observes that the groundwood pulp (GP) samples darkened extensively during exposure in the Fade-ometer� (Figure 1). Figure 3 suggests that the post-irradiation darkening [K/S90 days − K/Sinitial, Δ(K/S)] of the groundwood sheets was related to the amount of exposure. This relationship was not evident, however, in the case of the three papers that contained low levels of lignin. The slight degree of post-irradiation darkening of the UBP and BP samples [only one-tenth that of the groundwood pulp, GP, sheets when expressed as Δ(K/S)] was practically the same regardless of the irradiation dosage. Any trend in post-irradiation darkening in these papers relative to the amount of exposure would have been obscured by experimental errors owing to the small changes in reflectance that took place. The longest exposed sample of filter paper (FP) seemed to have darkened more than the other samples during storage; nonetheless, the net change in all test sheets of this paper was too small to permit a firm conclusion (Figure 2b). The almost
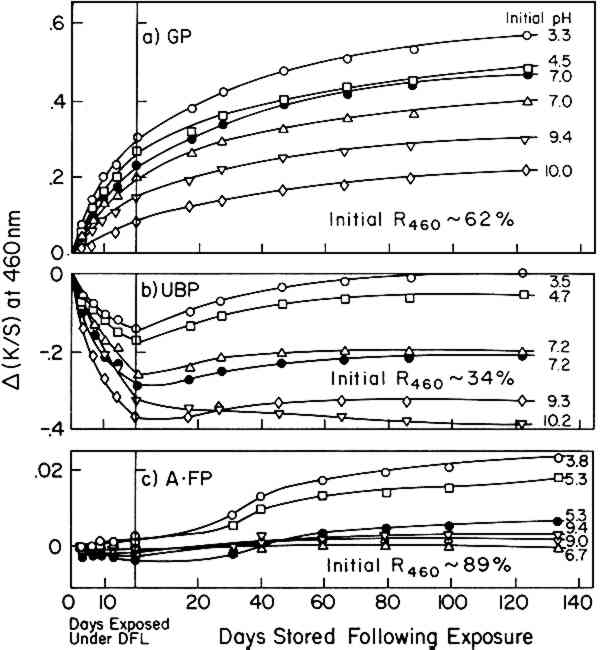
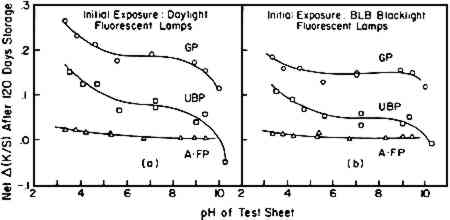
On first inspection of the major differences in composition of the papers (Table II), it appears that the degree of post-irradiation darkening following exposure in the Fade-ometer� might be influenced by the content of hot-alkali-soluble (HAS) matter (a measure of the oxidized and low-molecular-weight fractions of the pulp) as well as the lignin content in the paper. In this sense, the post-irradiation darkening might be considered to exhibit characteristics that one could expect from thermal-aging tests carried out on papers that have different degrees of oxidation as traditionally represented by carbonyl-group content, a property commonly reflected in the measurement of copper number or hot 1%-alkali-soluble (HAS) matter. This is essentially the conclusion reached by MacMillan using chemically-oxidized cotton linters.7 This point of view will be further considered in a later section in which handsheets made from pulps derived from groundwood were prepared in which both lignin content and HAS matter were varied. Probably both lignin and HAS content have an influence on post-irradiation darkening, but in the experiments described here HAS matter had little or no effect. 2.2 Effect of Initial pHAcidity greatly influences the thermally-induced deterioration of paper; how does acidity affect the post-irradiation darkening? Using handsheets prepared from two types of pulp (UBP, GP) and aged filter paper (A�FP, Whatman No. 42 filter paper thermally aged at 90�C and 50% RH for 150 hours), post-irradiation discoloration was tested as a function of initial acidity of the sheets having pH values from 3.2 to 10.2 as determined by cold-water extraction. Air-dried test sheets at different pH's were prepared by treating the sheets with various buffers or with calcium hydroxide solution (see Table III). “Daylight” fluorescent and BLB blacklight fluorescent lamps were used in order to observe the effects of both visible radiation (having a trace of near,
TABLE III Buffer Solutions Used for Paper Treatment∗ The UBP sample, an initially dark-colored pulp, bleached during exposure to the “daylight” fluorescent lamps; the acid sheets bleached the least. During subsequent storage, the UBP sheets tended to darken, the extent likewise being a function of the pH. As seen in Figure 4b, the alkaline-buffered papers exhibited the least post-irradiation darkening; at pH 10.2 the sheet apparently continued to bleach. Much the same results were observed with the UBP sheets during and following exposure to BLB blacklight fluorescent lamps, which emit principally near-ultraviolet radiation. The net post-irradiation change in the aged filter paper (A�FP) was an order of magnitude lower but tended to follow much the same trend (Figure 4c). When the net change in K/S at 460 nm after 120 days' storage, Δ(K/S), taken from Figure 4, is plotted against initial pH in the paper, Figure 5a results. In the case of exposure to BLB blacklight fluorescent lamps (near ultraviolet), the same general behavior is seen (Figure 5b). Although there are deviations in the experimental results, a general trend is indicated; post-irradiation darkening tends to be markedly reduced when the alkalinity of the sheets is above pH 9 and the degree of darkening increases when the papers have acidities greater than about pH 5. When one considers that pH is a function of the logarithm of the acidity or alkalinity, it is perhaps not unexpected that the accelerating effects at low pH would sharply curve upward and the minimizing effects of alkalinity would curve sharply downward in the linear plot of Figure 5. In the case of paper that contained little lignin, A�FP, acidity and alkalinity had scarcely any effect on the post-irradiation darkening. Again, one observes that the general level of darkening of the three types of paper, in the near-neutral condition, is in the order of the lignin content.
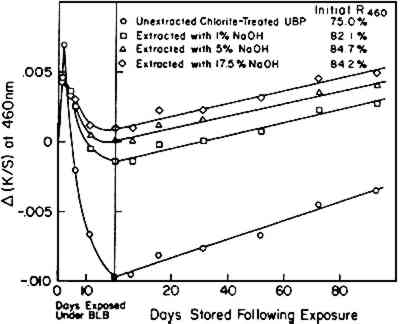
These results are simply technical observations regarding what happens to the papers having these values of pH when they are exposed to visible and near-ultraviolet radiation while on exhibition and are not at this stage intended to suggest changes in conservation-treatment procedures. 2.3 Effect of Gamma Cellulose ContentBulk material was subjected to a short period of chloriting to remove much of the original lignin content from the unbleached pulp (UBP) (about 4% initially, diminished by this treatment to less than 1%). Caustic extraction at different strengths of alkali solution was then employed to reduce the level of gamma cellulose in individual samples. The composition of the resultant pulps is reported in Table IV; previous experiments using these same pulps have been described elsewhere.14 TABLE IV Characterization ofr Caustic-Extracted Pulps Prepared from UBP Stock The post-irradiation effect on handsheets made from the different alkali-extracted UBP samples was investigated following exposure to the near-ultraviolet radiation from BLB blacklight fluorescent lamps. Exposures were carried out in the constant-temperature and -humidity room at 23�C and 50% RH for 19 days. Near-ultraviolet was used because it was considered that this radiation would provide a more severe exposure than daylight fluorescent lamps and therefore would enhance any detrimental effects of exposure that might be observed. The changes in K/S values at 460 nm that occurred during exposure to the BLB lamps and during subsequent storage at room temperature of 23�C and 50% RH are shown in Figure 6. During the short exposure of about 30 hours to the near-ultraviolet radiation of the BLB blacklamps, all samples rapidly, although slightly, darkened. Thereafter during the period of exposure, samples that had been extracted with alkali, which had low gamma cellulose content (8.8% or lower), tended to bleach, returning essentially to the K/S values at 460 nm that they had before exposure. The original sample of UBP (unextracted) also bleached extensively and, during storage, did not darken sufficiently in 90 days to return to its original K/S value.
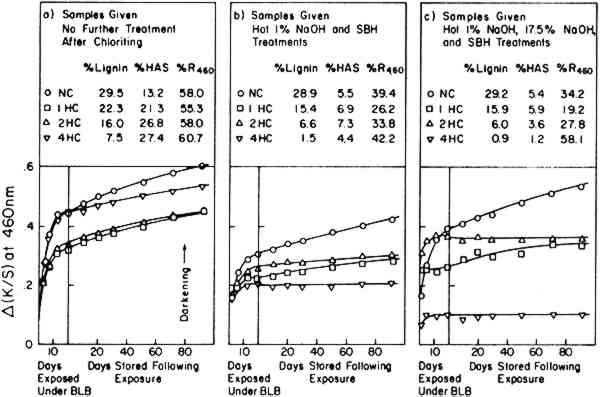
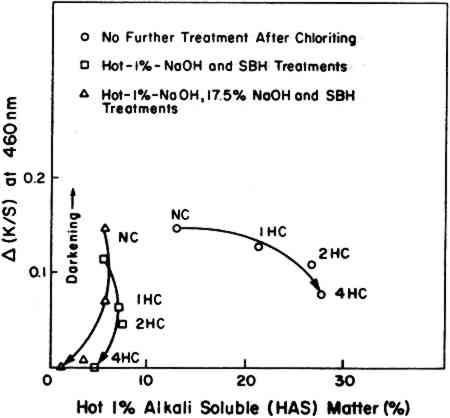
Note that the very small change observed in these tests (the right-side curves in Figure 6) exhibited almost the same rate of post-irradiation darkening irrespective of the composition of the extracted papers or the extent of the bleaching that had taken place under the BLB lamps. The test sheets contained little lignin; the percentage The results demonstrate, once again, that the post-irradiation tendency to darken, measured at 460 nm, is largely independent of the bleaching or darkening effects that may have occurred during exposure. 2.4 Effect of Lignin Content and Initial Hot-1%-Alkali-Soluble (HAS) MatterAs a final experiment, a variety of pulp samples were prepared from the groundwood pulp stock (GP) to allow the content of lignin and HAS matter to be varied as the result of a chloriting treatment followed by hot-1%-alkali extraction and by extraction with 17.5% sodium hydroxide solution. To minimize the possible effects of unintended oxidation of the cellulose, all samples were reduced with sodium borohydride prior to exposure. The values of initial lignin content and initial HAS matter in these samples are noted in Figure 7 where NC stands for not-chlorite-treated
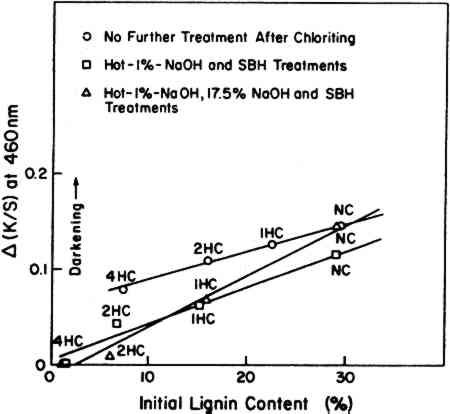
Twelve test sheets made from pulps thus variously prepared were exposed under the BLB lamps for 400 hours and then left in the dark at a room temperature of 23�C, 50% RH for up to 90 days to check post-irradiation darkening. It is clearly seen in Figure 8 that the post-irradiation darkening of these pulps is related to lignin content. This supports the general conclusion indicated by the Fade-ometer� exposures shown in Figure 1 versus Figure 2. When the results are compared to the initial content of HAS matter, in Figure 9, there is no indication that HAS content plays a major role in post-irradiation darkening.
3 CONCLUSIONSTHE RESULTS IN FIGURES 1, 2, 4, 6, and 7 demonstrate that, regardless of whether darkening or bleaching took place during exposure to visible or near-ultraviolet radiation, papers darkened upon subsequent storage in the dark over three-month's time at normal room conditions. For a high-lignin-content (29.1%) groundwood pulp, the extent of post-irradiation darkening after 90 days' storage increased with increased initial exposure in a xenon-arc Fade-ometer� (Pyrex� filters) (Figure 3). In contrast, post-irradiation darkening was little affected by the amount of initial exposure in the Fade-ometer� in the case of test sheets made from pulps that contained 4.4% lignin or less. In a series of experiments in which exposures were carried out at temperatures of about 25 to 28�C, the extent of initial darkening or bleaching and also the extent of post-irradiation darkening was found to be influenced by the pH of the test papers. Acid papers tended to darken the most upon subsequent storage and highly alkaline papers the least (Figure 4). The post-irradiation darkening of filter paper that had been previously thermally aged was scarcely affected by the pH of the test sheets (Figure 5). In an especially-prepared series of pulps, post-irradiation darkening was found to be directly related to lignin content over the range between 0.9 to 29.5% lignin (Figure 8). This result confirms the trend of relative darkening noted in papers containing 29.1, 4.4 and less than 0.24% lignin which were exposed in a Fade-ometer� (Figures 3 and 5). The presence of varying amounts of HAS matter was found to have no positive correlation with post-irradiation darkening (Figure 9). With a series of test sheets containing little lignin but varying amounts of gamma cellulose (Figure 6), little darkening was induced subsequent to exposure to near-ultraviolet radiation. 4 EXPERIMENTAL4.1 Preparation of Paper at Various pH ValuesTHREE TYPES OF WATER-LEAF TEST SHEETS—based on a supply of groundwood pulp (GP) and unbleached pulp (50% hardwood kraft/50% softwood kraft) (UBP), as well as unaged (FP) and aged filter paper (A�FP, Whatman No. 42 filter paper, previously aged 150 hours in an oven at 90�C, 50% RH)—were pretreated in various Table III presents the list of the buffers used to produce the sheets at various pH values. The resultant water-extracted pH values, measured on the sheets that had been dipped into these solutions and air dried, were not the same pH as that of the buffer solutions used to prepare the test sheets. The cold-water-extract pH values of the test sheets as finally achieved in the sheets are those employed in Figures 4 and 5. 4.2 Preparation of Pulps With Various Contents of Gamma CelluloseThe unbleached pulp stock was subjected to a brief chloriting treatment followed by an extraction with different strengths of alkali solution to remove the lignin and gamma cellulose.15 Twenty-five grams of sodium chlorite and 12.5 ml of glacial acetic acid were reacted at a temperature of 75 to 80�C for 30 minutes at a pulp consistency of 10% using 105g air-dried pulp. This treatment reduced the lignin content of the pulp. Caustic extractions were subsequently performed at a pulp consistency of 2%, with the concentrations of alkali, reaction temperature, and reaction time varied, as noted in Table IV. After the caustic extractions, the pulp was filtered off (except the 17.5%-alkali treated pulps, which were first diluted with water and then decanted), 500 ml of 10% acetic acid added and the mixture allowed to soak for 1 minute before removal of the pulp. Treatment with acetic acid was repeated with a second 500 ml portion. The pulp was then washed several times until the wash water tested acid-free with pH paper. Handsheets were made from the resulting pulps at a basis weight of 75 g/m2, as other types of handsheets. Alpha, beta, and gamma cellulose in the resultant pulps was determined by standard Tappi methods as noted in a previous publication.14 4.3 Pulp Treatments to Produce Variable Lignin Content and HAS MatterTo prepare the pulp at various contents of lignin, groundwood pulp (designated as NC, not chlorited) was subjected to chloriting, using the technique of Wise, et al. 15 for 1, 2, and 4 hours, respectively (yielding stock samples 1HC, 2HC, and 4HC). Further treatments were carried out with hot-1%-alkali solution in order to remove the degradation products arising from chloriting, and with 17.5% caustic soda to remove the hemicellulose component. Thereafter, these pulps were treated with 2% sodium borohydride (SBH), at 2% pulp consistency and room temperature for 20 hours with occasional stirring, to convert aldehyde groups to hydroxyl. Lignin content and HAS matter were determined on the resultant pulps by Tappi methods T222 os-74 and T212 os-76, respectively. 4.4 Exposure to Light SourcesThree different light sources were used: (a) a bank of six General Electric High-Output (HO) “daylight” fluorescent lamps, (b) a bank of six fluorescent BLB blacklights, and (c) an Atlas 6500-W xenon-arc Fade-ometer� with Pyrex� filters. Data on the irradiance under the three lamps is given in Table V, information provided primarily to indicate the percentage of ultraviolet emitted. TABLE V Irradiance From Different Light Sources (mw/cm2) Both types of fluorescent lamps were mounted about 3 1/2″ above the samples in a room maintained at 50% relative humidity (RH) and 23�C. The temperature of the handsheets reached about 27�C. In the 6500-W Fade-ometer� the air was maintained at 31�C (�1.0�) and 27% RH; the black-panel temperature reached 65�C. 4.5 Storage ConditionsFollowing exposure to the various light sources, samples were kept in the closed steel cabinet in the paper-testing room maintained at 23�C and 50% RH. REFERENCESR. A.Stillings and R. J.Van Nostrand. “The Action of Ultraviolet Light upon Cellulose.”J. Am. Chem. Soc., 661944, pp. 753–760. H. F.LaunerW. K.Wilson. “The Photochemistry of Cellulose. Effects of Water Vapor and Oxygen in the Far and Near Ultraviolet Regions.”J. Am. Chem. Soc., 71, 1949, pp. 958–962. G. S.Egerton. “The Mechanism of the Photochemical Degradation of Textile Materials.”J. Soc. Dyers and Colourists, 65, 1949, pp. 764–780. R. H.MacClaren, F. L.Wells, J. V.Rosequist, and D. F.Ingerick. “Brightness Reversion of Cellulose Exposed to Ultraviolet Light.”Tappi, 45, 1968, pp. 789–793. This subject has been reviewed by R. L.Feller, S. B.Lee and J.Bogaard, “The Darkening and Bleaching of Paper by Various Wavelengths in the Visible and Ultraviolet,” Postprints, The Book and Paper Group, American Institute for Conservation of Historic and Artistic Works, the tenth annual meeting, Milwaukee, Wisconsin, 1982. pp. 26–30. H. F.Launer and W. K.Wilson. “Photochemical Stability of Papers.”J. Research National Bureau of Standards, 30, 1943, pp. 55–74. W. R.MacMillan, M.A. Sc.Thesis, “Color Reversion in Oxidized Cellulose.” Department of Chemical Engineering and Applied Chemistry, University of Toronto, Canada, 1958. H. W.Giertz. “The Yellowing of Pulp.”Svensk Papperstid., 48, No. 13, 1945, pp. 317–323 For further discussion of the relationship of Tappi brightness and the use of K/S values see R. L. Feller, “Comments on the Measurement of ‘Yellowness’ in Pulp and Paper”, The Book and Paper Group Annual (IIC), 6, 1987, pp. 40–51. The expression “brightness reversion” has often been used for darkening that occurs after various treatments that lead to brightening. We are not in favor of this terminology because, if one component is bleached during a treatment, and another component is responsible for the subsequent discoloration, the paper may not have reverted to its original color or composition of chromophoric groups. It is important for the research laboratory to begin to ask: what is responsible for the color before treatment and what is responsible for the change that occurs as the immediate result of treatment, that which arises after treatment? Handbook of Chemistry and Physics,The Chemical Rubber Co., Cleveland, Ohio, 1983, p. D–155 (63rd Edition). Tappi Standard T509. M.Hey. “The Washing and Aqueous Deacidification of Paper”, The Paper Conservator, Vol. 4, 1979, pp. 66–80. S. B.Lee and R. L.Feller. “Influence of the Hemicellulose Fraction on Thermal and Photochemical Discoloration of Paper.”Advances in Chemistry Series, No. 212, Washington, D.C., ACS, 1986, pp. 377–386. L. E.Wise, M.Murphy and A. A.D'Addieco, “Chlorite Holocellulose, Its Fractionation and Bearing on Summative Wood Analysis and on Studies on the Hemicelluloses.”Paper Trade J., 122, No. 2, 1946, pp. 35–43.
 Section Index Section Index |