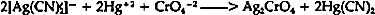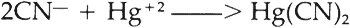TREATMENT OF A SILVER DRAGON FOR THE REMOVAL OF SILVER CYANIDE AND CHALCONATRONITEDonna K. Strahan
APPENDIX1 APPENDIX1.1 SPOT TEST FOR SILVER CYANIDE THROUGH CONVERSION INTO SILVER CHROMATE16“The conversion of silver cyanide into silver chromate provides a sensitive and specific test for AgCN because other water-insoluble, acid-resistant silver salts such as AgCl, AgBr, AgI, AgSCN, AgIO3, etc. do not react. Procedure: A small amount of the finely divided sample is placed on a piece of filter paper or in a depression of a spot plate and spotted with a drop or two of the reagent. The formation of a red-brown color (silver chromate) indicates the presence of silver cyanide. Reagent: MercuryII nitrate (or acetate)-potassium chromate solution. Several ml of a 5% aqueous solution of mercuric nitrate or acetate are made acidic with dilute acetic acid and 2 g of sodium acetate are added, followed by several drops of a potassium chromate solution. Should a slight precipitate appear, it can be removed by filtering or centrifuging. The clear yellow reagent is stable. The conversion of AgCN to AgCrO4 by means of the reagent also occurs in solutions containing complex [Ag(CN)2]− ions.
An excess of alkali cyanide causes no interference because CN−ions are removed by the formation of mercuric cyanide
The above procedure can be used for the detection of silver cyanide in ammoniacal solution. However, preliminary evaporation to dryness is an essential first step.” NOTES1. X-ray diffraction analyses were performed by Paul Jett of the Technical Laboratory of the Freer Gallery of Art and also by Martha Goodway of the Conservation Analytical Laboratory, Smithsonian Institution. 2. Strahan, p. 63. 3. X-ray fluorescence and infrared spectrophotometric analyses were performed by the Martin Marietta Corporation of Baltimore, Maryland. 4. Dick, p. 297. 5. Hiorns, p. 85. 6. Kirk-Othmer, p.64. 7. Personal communication with Don Heller, Conservation Department, Winterthur Museum, Winterthur, Delaware. 8. Committee on Hazardous Substances in the Laboratory, p. 147. 9. Gettens and Frondel, p. 64. 10. Horie and Vint, p. 185. 11. Untracht, p. 686. 12. Correspondence with Dr. F. Christopher Tahk, Director, Art Conservation Department, State University College at Buffalo, Cooperstown, New York. 13. X-ray diffraction analysis was performed by the author with the guidance of Joan Mishara of the Conservation Analytical Laboratory, Smithsonian Institution. 14. Curtman, p. 377. 15. Committee on Hazardous Substances in the Laboratory, p. 46. 16. This appendix is taken verbatim from Feigl, pp. 352–353. BIBLIOGRAPHYBaekeland, Frederick. Imperial Japan: The Art of the Meiji Era (1868–1912), Cornell University, New York, 1980. Committee on Hazardous Substances in the Laboratory. Prudent Practices for Handling Hazardous Chemicals in Laboratories, National Academy Press, Washington, D.C., 1981. Curtman, Louis J.Qualitative Chemical Analysis, The Macmillan Company, New York, 1938. Dick, William B.Dick's Encyclopedia of Practical Receipts and Processes or How They Did it in the 1870's, Funk & Wagnalls, New York. Feigl, Fritz and Anger, Vinzenz. Spot Tests in Inorganic Analysis, Elsevier Publishing Company, New York, 1972. Gettens, Rutherford J., and Frondel, Clifford. Chalcontronite: An Alteration Product on Some Ancient Egyptian Bronzes. Studies in Conservation, 2,21955, pp. 64–74. Hiorns, Arthur H.Metal-Colouring and Bronzing, Macmillan and Co., New York, 1892. Horie, C. V., Vint, J. A.Chalconatronite: A By-Product of Conservation? Studies in Conservation, 27, 1982, pp. 185–186. Hughes, Richard and Rowe, Michael. The Colouring, Bronzing and Patination of Metals, Crafts Council, London, 1982. Kirk, Raymond E. and Othmer, Donald F.Encyclopedia of Chemical Technology, 8, John Wiley and Sons, New York, 1979. Oguchi, Hachiro. Experimental Studies on Japanese Traditional Coloring Copper. Bulletin of the Faculty of Fine Arts Tokyo University of Arts, 8, Tokyo, 1972. Oguchi, Hachiro. Japanese Shakudo: Its History, Properties and Production from Gold-Containing Alloys. Gold Bulletin, 16, 4, 1983, pp. 125–132. Roberts-Austen, W. Chandler. Cantor Lectures: Alloys. Journal of the Society of Arts, November 20, 1893, PP. 1022–1030. Savage, E. and Smith, C. S.The Techniques of the Japanese Tsuba Maker. Ars Orientalis11, 1979, pp. 291–328 Strahan, Donna K.A Cautionary Note on the Presence of Silver Cyanide on Museum Objects. Journal of the American Institute for Conservation23, 1983, pp. 63–64. Untracht, Oppi. Jewelry Concepts and Technology, Doubleday and Co., New York, 1982. |