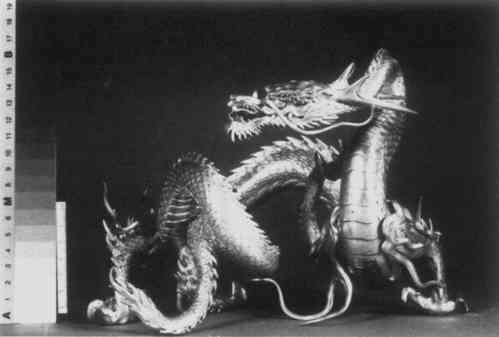
TREATMENT OF A SILVER DRAGON FOR THE REMOVAL OF SILVER CYANIDE AND CHALCONATRONITEDonna K. Strahan
3 COMMERCIAL SILVER CYANIDE CLEANINGA CURATOR RECALLED that the dragon had been sent to a commercial silver company for cleaning during the early 1960's. The silver company to which the dragon was sent is no longer in existence, and their method of cleaning silver is not known. Since the mid-19th century, immersion in a cyanide bath has been a common method for removing silver tarnish, as well as for plating silver. The existence of cyanide corrosion on the surface of the dragon leads to the belief that it was cleaned by the above method. A probable scenario is that the cyanide solution entered the hollow sections of the body through minute gaps in joints or flaws in the casting. Some of the solution may have remained on the surface of the interior due to poor rinsing and then slowly migrated to the exterior surface, attacking the silver and forming cyanide salts. The interior has probably been corroded in a similar manner, but there is currently no means to confirm this without damaging the object. There are numerous old recipes for cleaning silver which call for the use of a potassium or sodium cyanide solution. For example, Dick's recipe4 calls for 1 gallon of water, 4 ounces of potassium cyanide, 1 pound of hyposulphite of soda (sodium thiosulfate), 8 ounces of muriate of ammonium (ammonium chloride) and 4 ounces of liquid ammonia (ammonium hydroxide). In 1892 Hiorns discussed removing silver sulphide with a five percent solution of potassium cyanide.5 Modern formulations differ very little from earlier baths. They include potassium cyanide, sodium cyanide and sodium carbonate.6 Cleaning in a modern commercial silver shop is often begun by degreasing the object in a potash or lye solution and rinsing it in water. If copper corrosion is present on a silver alloy, commercial shops often remove it with sulphuric acid or hydrochloric acid before dipping the object into a cyanide solution. It is dipped into a ten percent cyanide solution, followed by a water rinse. Cyanide salts deposited from immersion in the bath are removed with a scratch brush, a fine brass wire brush described as soft as human hair. The object is then polished with a lye compound and rouge.7
|