CONSOLIDATION OF DETERIORATED WOOD WITH SOLUBLE RESINSY. Wang, & A.P. Schniewind
5 EXPERIMENTAL MATERIALS AND METHODSDOUGLAS-FIR FOUNDATION piles (long, heavy timbers, usually round, driven into soft soil to provide a secure foundation), which had been in service a short distance inland from the San Francisco waterfront for about 70 years, were chosen as the sample source. The first 25 mm from the pile surface had been found to be severely degraded by bacteria (Schniewind, et al., 1982). This material was chosen because it was a readily available source with a known cause and extent of deterioration. The piles had been water-logged while in service, but had been allowed to air-dry after extraction and removal. Since only the most severely deteriorated material was desired, specimens were cut from the outermost layer (first 4 to 5 mm) of a section of each of four piles. Static bending tests of matchstick-size specimens were selected to evaluate improvements in strength and stiffness. Three by 3 by 50 mm bending specimens, as previously used by Schniewind and Kronkright (1984), were prepared. All specimens were conditioned to a nominal moisture content of 12% at 21�C and 65% relative humidity before treatment and prior to testing. These are standard conditions for wood testing. The authors believe that similar results would be obtained by testing at, say, 50% relative humidity.
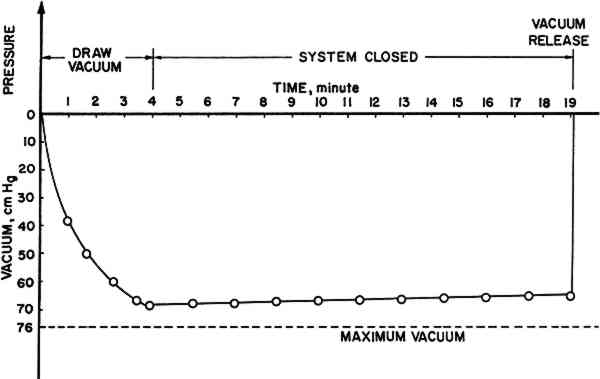
Schniewind and Kronkright (1984) found that polyvinyl butyral (Butvar) gave the most improvement in strength, followed by acrylic (Acryloid B72). Accordingly, these commercially available thermoplastic polymers were further investigated in this study. Two different molecular weights of polyvinyl butyral, Butvar B90 and B98, were each dissolved in two solvents. For Acryloid acrylic resins, which are copolymers of acrylic and methacrylic acid and their esters, only one molecular weight of resin (Acryloid B72) was tested in two solvents. The solvents chosen for polyvinyl butyral were 95% ethanol and a mixture of 40% ethanol and 60% toluene on a weight basis. For Acryloid B72, acetone and toluene were used as solvents. For each resin and solvent combination, three resin concentration levels were chosen. These were 5.0%, 12.5%, and 20.0%. Three resins, two solvents, and three concentration levels provided eighteen different treatments. Another set of the same resin and solent combinations at 12.5% concentration was prepared for testing the effect of using a very slow rate of solvent removal. This added another six treatments. For each treatment, there were 20 replications, 5 specimens from each of 4 piles. This gave a total of 480 specimens. Another 100 specimens, 25 specimens from each of the 4 piles, made up a control (untreated) group. Thus, there were 580 specimens in this study. After dissolving each polymer in its respective solvent, the specimens were submerged in the resin solution in a small dish which was covered with aluminum foil to prevent solvent evaporation. The specimens were allowed to soak in the respective resin solution for two hours before vacuum impregnation. The dish was then placed inside a desiccator, which served as vacuum chamber, followed by 19 minutes of vacuum using a tapwater aspirator. The aspirator was adjusted to produce a vacuum of about 68 to 69 cm Hg, as determined by a mercury manometer, in four minutes, and then the system was closed. This was done to minimize evaporation of solvent and resultant increases in resin concentration. In addition, another dish containing pure solvent was placed inside the desiccator to assist in saturating the space inside the desiccator with solvent vapor. The vacuum condition vs. time is shown in Fig. 1.
For Acryloid B72, the vacuum could only reach 66.5 cm Hg, instead of 68 cm Hg, since the solution started boiling at 67 cm Hg, the specimens acting like boiling chips in facilitating nucleation of bubbles. Boiling was considered undesirable because the rapid vaporation might hasten changes in concentration. At the end of the vacuum period, air was introduced, and the specimens were allowed to soak in the solution for another 30 minutes. Then they were taken out of the solution and blotted with paper toweling. The majority of the specimens were set out to dry in a room held at 65% relative humidity and 21�C. The evaporation of solvent usually took place rapidly within the first day under the conditions stated above. For slow drying, specimens were dried in weighing bottles which were opened only briefly each day for 60 days. All specimens were left to equilibrate at 65% relative humidity According to Schniewind and Kronkright (1984), no significant increase in retention was found when the vacuum process (evacuation followed by release of pressure) was repeated. Therefore, the vacuum cycle was carried out only one time. The static bending test was carried out on a table model Instron testing machine, over a span of 42 mm and with a loading speed of 0.01 inch per minute. Since the specimens are very small, bending strength might be influenced by whether latewood or earlywood bands happen to be at the top and bottom surfaces if load is applied to the tangential face. In order to avoid this, load was applied to the radial surface to obtain more reliable and uniform results. Values of modulus of rupture (blending strength) and modulus of elasticity (bending stiffness) were calculated for the treated and untreated specimens from the recorded load-deflection curves, followed by statistical analyses using a library computer routine called Statistical Analysis System (SAS package). |