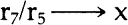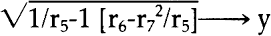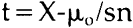SOME OBSERVATIONS ON THE USE OF ENZYMES IN PAPER CONSERVATIONPia C. DeSantis
NOTES1. A. L. Lehninger, Biochemistry, Worth Publishers, Inc., New York, 1972, pp. 55–56. 2. Ibid., p. 62; pp. 169ff. See also S. Blackburn, Enzyme Structure and Function, Marcel Dekker Inc., New York, 1976; C. Walsh, Enzymatic Reaction Mechanisms, W. H. Freeman and Co., San Francisco, 1979; A. Fersht, Enzyme Structure and Mechanism, W. H. Freeman and Co., San Francisco, 1977. 3. See Lehninger, op. cit.(note 1), pp. 169ff. for a good section on general terminology. 4. See Blackburn, op. cit.(note 2), pp. 497f; for excellent diagrams, see Lehninger, op. cit.(note 1), p. 176; also S. Kindel, “Enzymes: the Bioindustrial Revolution”, Technology, November/December 1981, esp. pp. 64, 66–67. I would like to thank Jerri Nelson for calling my attention to this article. 5. See Lehninger, op. cit.(note 1), pp. 169ff.; also Blackburn, op. cit.(note 2), p. 497. 6. See Walsh, op. cit.(note 2); Lehninger, op. cit.(note 1), pp. 169ff. 7. Dixon and Webb, Enzymes, third edition, Academic Press, New York, 1979, p. 11. Also see the sources cited in note 2; for a succinct approach to this enzyme characteristic, see D. Grattan, J. St. Hilaire, H. Burgess and C. McCawley, “The Characterization of Enzymes for use in Paper Conservation”, The Conservation of Library and Archive Materials and the Graphic Arts, The Institute of Paper Conservation, Cambridge, 1980. 8. Lehninger, op. cit.(note 1), pp. 169ff.; Dixon and Webb, op. cit.(note 7); Kirk-Othmer, Encyclopaedia of Chemical Technology, third edition, John Wiley and Sons, New York, 1980, v. 9. 9. Grattan et al, art. cit.(note 7). 10. M. A. Johnson, personal communication, 10/8/83. 11. Walsh, op. cit.(note 2), chapter 3; see also Lehninger, op. cit.(note 1), pp. 169ff. 12. A synthetic bristle brush should be used when employing a protein digesting enzyme. 13. M. Hatton, “Enzymes in a Viscous Medium”, The Paper Conservator, v. 2, 1977. 14. D. Cooper, C. King and J. Segal, “The Use of Enzymes in Partially non-Aqueous Media” in The Conservation of Library and Archive Materials and the Graphic Arts, Institute of Paper Conservation, Cambridge, 1980. 15. M. A. Johnson does not suggest using enzymes in solvents in paper conservation applications since the enzymes will coagulate and thus be difficult to remove from the paper. M. A. Johnson, personal comunication, 10/8/83. 16. P. Banks, “Paper Cleaning”, Restaurator, v. 1, no. 1, 1969, p. 56. 17. O. Wendelb⊘ and B. Fosse, “Protein Surgery: A Restoring Procedure Applied on Paper”, Restaurator, v. 1, no. 4, 1970. 18. J. Segal and D. Cooper, “The Use of Enzymes to Release Adhesives”, The Paper Conservator, v. 2, 1977, pp. 47–50. 19. Ibid. 20. Grattan et al, art. cit.(note 7). 21. H. Burgess and C. Charette, “Aspects of Image Safety in the Use of Enzymes in Paper Conservation” ICOM Committee for Conservation, 6th Triennial Meeting, Ottawa, 1981, 81/14/10. 22. See for example, S. Fletcher and J. Walsh, “The Treatment of Three Prints by Whistler on Fine Japanese Tissue”, Journal of the American Institute of Conservation, v. 18, #2, 1979, pp. 118–126; D. Cooper, C. King and J. Segal, “The Use of Enzymes in Partially non-Aqueous Media”, and W. van Oort and P. Poldervaart, “Prints Off the Ice: The Conservation and Restoration of the Nova Zembla Prints”, both in The Conservation of Library and Archive Materials and the Graphic Arts, Institute of Paper Conservation, Cambridge 1980; Hatton, “Enzymes in a Viscous Medium”, The Paper Conservator, v. 2, 1977; O. Wendelb⊘, “The Freeing of Papyri from Cartonnage,” Restaurator, v. 2, no. 2, pp. 41–52. There has been a comparative examination of enzyme treated papers with untreated samples using the scanning electron microscope: P. Flood and O. Wendelb⊘, “The Enzymatic Extraction of Papyri from Cartonnage: 23. Segal and Cooper, art. cit.(note 18), “Acknowledgements”, p. 59; J. Segal, personal communication, 5/14/81. 24. Nina Rayer, personal communication, 4/83. 25. Segal and Cooper, art. cit.(note 18), pp. 47–48. 26. Grattan et al, art. cit.(note 7), pp. 7, 10. It is also noteworthy that the reaction rate of a proteolytic enzyme will begin to decrease after a certain period due to autodigestion (that is, a protease will catalyse its own breakdown since it too is a protein); see for example, Dixon and Webb, op. cit.(note 7), chapter 1. Grattan et al point out as well that the by-products of enzymatic reactions can “poison” the enzyme, and thereby slow the initial reaction rate (art. cit.[note 7], p. 10). It should be noted that the autodigestion reaction should not happen too quickly since most enzymes are resistant to proteolytic attack. Boyer, op. cit. below, (note 32); p. 257. 27. See, for example, Dixon and Webb, op. cit.(note 7), pp. 139, 165; see pp. 226–227 for a concise statement of the conditions necessary for a successful enzymatic reaction. 28. Dixon and Webb, op. cit.(note 7), pp. 165–166. 29. Dixon and Webb, op. cit.(note 7), pp. 139–140; Lehninger, op. cit.(note 1), pp. 133, 158. 30. Ibid., p. 139. 31. Ibid., p. 136. It should be noted that it is not advisable to consider the option of using an excess of enzyme when planning a conservation treatment. 32. P. Boyer, ed., The Enzymes, third edition, Academic Press, New York, 1972; B. subtilis α amylase: v. V, pp. 235ff.; S. griseus protease (a DFP sensitive alkaline protease): v. III, pp. 744ff. 33. See above, note 26. 34. In tests run by the author, it was found that the protease derived from A.saitoi, also called Aspergillopeptidase A, functioned when a temperature of 37�C and a pH of 6 was maintained, although the enzyme's pH optimum is 2.5–3.0. See Boyer, op. cit.(note 32), v. III, pp. 722ff. 35. M. A. Johnson, personal communication, 10/8/83. 36. For a detailed list of additives, see Kirk Othmer, op. cit.(note 8), pp. 173ff. 37. Ibid.; see also trade journal articles such as E. Beckthorn, M. Labbee, L. Under-holfer, “Production and Use of Microbial Enzymes for Food Processing”, Agricultural and Food Chemistry, v. 13, Jan.–Feb. 1965, pp. 30–34. I am grateful to Catherine Nicholson for calling my attention to this article. 38. See, for example, the product descriptions in the Sigma Chemical Company Catalogue. 39. van Oort and Poldervaart, art. cit.(note 22); Burgess and Charette, art. cit.(note 21). 40. Boyer, op. cit.(note 32), v. III, pp. 744ff. 41. Kindel, art. cit.(note 4). 42. Wood and McCrae, “Synergism Between Enzymes Involved in the Solubilization of Native Cellulose”, in Brown and Jurasek, ed., Hydrolysis of Cellulose: Mechanisms of Enzyme and Acid Catalysis, Advances in Chemistry Series #181, American Chemical Society, Washington, D.C., 1979. For an explanation of cellulose behavior under synergistic attack by enzymes, see Cong, Ladisch and Tsao, “Biosynthesis, Purification and Mode of Action of Cellulases of 43. Millett, Effland and Caulfield, “Influence of Fine Grinding on the Hydrolysis of Cellulosic Materials: Acid versus Enzymatic” in Brown and Jurasek, op. cit.(note 42). 44. Ibid. 45. Vitale and Simeon, ed., Industrial and Clinical Enzymology, Pergamon Press, New York 1980. Any form of celluolose which retains at least some of its native crystalline structure will prohibit enzyme accessibility. 46. Dr. M. D. Appleman, personal communication, 5/4/83, suggests some unsophisticated but effective methods that one could use as an “in-house” check of the degree of purity of an enzyme preparation. To test an amylase, one could boil egg white until coagulated, take a tiny portion and place it in an amylase solution heated to 30–37�C. If the egg white remains unaltered, the amylase should be pure enough for our purposes. For a protease, one would perform the same test, only placing a small amount of boiled starch in a solution of protease heated to 30–37�C. If the egg white or starch were affected by these treatments, it would mean that the enzyme had not been properly purified. One would then have to question whether an impurity of cellulase was present. 47. Dr. Appleman is a Professor (retired) of the Microbiology Dept., USC. Dixon and Webb, op. cit.(note 7), p. 166. 48. Breed, Murray and Smith, Bergey's Manual of Determinative Bacteriology, The Williams and Wilkins Co., Baltimore, 1957. 49. Dr. M.D. Appleman, Professor (retired), Microbiology Department, University of Southern California, personal communication, 5/83. Mike Mahinka of Customer Services, Novo Laboratories had suggested the same idea, personal communication, 9/82. A primitive series of tests were run using the protease from A.saitoi in an attempt to determine whether 10 and 30 minute baths of ethanol and alkaline water could remove the enzyme from cotton. The results were encouraging, but not conclusive, since the protein detecting agent used is sensitive enough to detect the protein in cotton. See the procedure for ninhydrin test, B. Browning, Analysis of Paper, Marcel Dekker, Inc., New York, 1969, pp. 95–96. 50. See, for example, the molecular weights listed by Boyer, op. cit(note 32), v. III, Table VIII, pp. 756–757. 51. Ibid. It is noteworthy that all the α amylases have molecular weights of 40,000 to 60,000 amu. Boyer, op. cit.(note 32), v. V, p. 250. 52. Segal and Cooper, art. cit.(note 18), pp. 46–47. 53. Cooper, King and Segal, art. cit.(note 22), p. 17. 54. Ibid. 55. Dixon and Webb, op. cit.(note 7), p. 11: “Organic solvents, for example, alcohol, inactivate most enzymes at room temperature, except at low concentrations”. 56. Lehninger, op. cit.(note 1), p. 134. 57. Segal and Cooper, art. cit.(note 18). 58. See, for example, the discussion of inactivation of specific enzymes in Boyer, op. cit.(note 32), v. III, chapter 20; v. V, pp. 235ff.; Dixon and Webb, op. cit.(note 7), chapter 1; Cooper, King, Segal, art. cit.(note 22), p. 17. It is also note-worthy that organic solvents are used to separate proteins that need to survive this separation treatment with their properties unaltered: thus, the coagulation that such solvents cause must be reversible. See for example Lehinger, op. cit.(note 1), p. 134. M. A. Johnson, personal communication, 10/8/83, 59. These precautions should minimize any danger of reactivating residual enzyme since there is evidence that the possibility of such a reactivation is a matter of the percentage of residual enzyme. See Boyer, op. cit.(note 32), p. 254: “Taka-amylase A [A. oryzae α amylase] is one of the proteins that was found to be renatured from a random coil state. The presence of an optimum concentration for the refolding of reduced Taka-amylase A was observed”. 60. Lehninger, op. cit.(note 1), p. 59. 61. Lehinger, op. cit.(note 1), pp. 59–60; Dixon and Webb, op. cit.(note 7), p. 167; Boyer, op. cit.(note 32), v. V, p. 254, with bibliography of articles presenting research that confirms the recoiling of denatured enzymes. 62. M. A. Johnson believes that using a minimal amount of enzyme and rinsing thoroughly probably renders an inactivation step unnecessary. M. A. Johnson, personal communication, 10/8/83. 63. Segal and Cooper, art. cit.(note 18). 64. See Boyer, op. cit.(note 32), v. V, pp. 238–258. This treatment was recommended for the α amylase from B. subtilis (see note 63). 64. Boyer, op. cit.(note 32), v. V, pp. 247–249 (see pp. 253–256 for the resistance of this enzyme to denaturation); see also the sources cited below, note 61. Calcium may also make the enzyme less susceptible to inactivation by alcohol, although the author has not found a direct statement confirming this supposition. However, there is the comment in Boyer (op. cit.[note 32], pp. 247–8) that the bound calcium makes the enzyme less liable to changes in its environment. An environmental change would include ionization as well as temperature. 66. Ibid. 67. A hot water bath has been recommended to denature this enzyme as well. See Segal and Cooper, art. cit.(note 18), p. 48. 68. Boyer op. cit.(note 32), v. III, pp. 744ff. Also see above, note 65. 69. To given the reader a sense of the amounts involved: α amylase has three binding sites for calcium per molecule of enzyme; for a neutral protease weighting 40,000 g-atoms, 0.0033M calcium ion was needed for stability against heat; a 0.00025M solution of EDTA removed all of the calcium from a B.subtilis∗ amylase; 15 micrograms of CaCl2 were sufficient to obtain full activity for 0.6 ml of a calcium-dependent extracellular amylase at 40�C; B.subtilis α amylase needs four or more gram-atoms of calcium/mole enzyme for complete activity. For discussions of the mechanism of these phenomena, see K. Yutani, “Role of Calcium Ion in the Thermostability of Amylase Produced from Bacillus Stearothermophilus”, pp. 91–103; W. Heinen and A. Lauwers, “Amylase Activity and Stability at High and Low Temperature Depending on Calcium and other Divalent Cations”, pp. 77–89; K. Mizusawa and F. Yoshida, “Role of a Sulfhydryl group in the Structure and Function of Alkaline Proteases from a Thermophylic Actinomycete”, pp. 61–65, all in H. Zuber, ed., Enymes and Proteins from Thermophilic Microorganisms, Birkhauser Verlag, Basel, 1976. Also see Boyer, op. cit.(note 32), v. V., pp. 247f. 70. E. Ichishima, F. Yoshida, “Conformation of Aspergillopeptidase A [A.saitoi protease] in Aqueous Solution”, Nature, v. 128 (1966) pp. 130–135; Boyer, op. cit.(note 28), v. III, pp. 722ff. 71. Boyer, op. cit.(note 32). M. A. Johnson has noted that such preparations are more resistant to inactivation than most, and believes that the conservator should thus use a minimum percentage and only in cases where the object can withstand 72. As described above, section 3, pp. 4f. 73. Dr. M. D. Appleman, personal communication, 5/4/83. Another consideration would be whether the enzyme will break down into acidic byproducts, a question which the pH testing presented here hopes to begin to investigate. Theoretically, of course, enzymes are amphoteric compounds. It is interesting to note that the protease from A. saitoi showed 5% autodigestion after 16 hours in a solution of pH 5.5 and temperature 22–25�C (Ichishma and Yoshida, art. cit.[note 70]). Consideration of this data in the light of the findings of the unsophisticated experiments discussed above, pp. 10–11, suggest the importance of moisture to enzyme breakdown, at the least, in enzyme breakdown due to autodigestion. 74. Boyer, op. cit.(note 32), v. III, pp. 723f. 75. See above, note 70. 76. The author is grateful to M. A. Johnson for these suggestions. Since the enzymes used by conservators are quite water soluble but often difficult to inactivate, he surmises that the best procedure is to use the least percentage of reagent possible and rinse well. The theories proposed for the mechanism of enzyme reactions suggest that in the case of an undenatured enzyme, the enzyme's potential bonding sites will be interacting with appropriate sites on the water molecules and the preferred substrate. In a system of proteolytic enzyme, water, glue and paper, then, paper should not compete well with glue and water for the enzyme's binding sites. (The same conditions apply in a system of starch paste and starch digesting enzyme.) It seems reasonable to assume that as long as an enzyme remains stable during treatment and rinsing and is used in small quantities, it should not tend to stay in the paper and should be easy to remove. 77. Bob Futernick has suggested the use of radioactivity tagged enzymes to study paper's retention of these reagents (personal communication, 5/1983). The author is currently pursuing examination of her samples under the Scanning Electron Microscope. 78. See above, note 70. 79. Ibid. 80. Ibid. Indeed, 10 minutes in a pH 7.8 solution of deionized water passed through a column containing calcium and magnesium inactivated this enzyme. The author lacked the reagents and equipment to determine whether the inactivation was irreversible. In at least some cases, incorrect folding of the enzyme is what causes irreversible denaturation. Boyer, op. cit.(note 32), v. V, p. 256. 81. Discovered through testing the enzyme on a Rives paper coated with gelatine that had been over aged at 100�C for three days. The pH of the enzyme bath was six. It was found that for a satisfactory reaction rate, it was necessary to maintain a temperature of 37�C. A 0.08% solution that was five weeks old (stored in the refrigerator) still attacked the aged gelatine when heated to 37�C. (Pure water heated to this temperature had no effect in the same time period.) 82. Tests were not performed on a system of paper and media, since the protease used by Burgess and Charette (art. cit.note 21) attacks a broader range of substrates and is more reactive than that derived from A.saitoi. 83. Aquabee Newsprint Rough 887-R, Bee Paper Co. Inc., Passaic, New Jersey, sold in 8″ � 11″ pads. 84. Rives “Infinity”, Lightweight, white, 115g/m2, sheets cut 19 � 26″. Unfortunately, no data as to the nature of fillers or sizing could be obtained from the United States distributors of this paper. 85. The Paper Conservation Lab of the National Gallery of Art, Washington, D.C., uses the Culligan D series deionizer column with a weak base anion resin. The water will “typically contain less than 0.5 mg/1 of dissolved minerals” (Culligan Product Literature). Before coming through the tap, the water is passed through a particulate material column. If alkalinity is desired, the water can also be diverted through a column containing marble chips and magnesium, usually in the hydroxide form. Water from the deionizer was used for all solutions in this series of experiments, and water from the alkaline column was used for all the rinse baths. 86. See note 85. 87. To safeguard the distilled water from absorbtion of carbon dioxide from the atmosphere, Barbara Miller of the Analytical Lab, National Gallery of Art, devised a method of storing the water under soda lime. A tube was equipped with open cone stoppers at either end. These stoppers were plugged with angel hair, and the tube was filled with chips of soda lime. The apparatus was fixed into the center hole of the cork used to plug the water container. It may be of interest to other researchers that a 1 gram sample of paper is not essential to meet TAPPI standard T509 os77, as long as the specified ratio of water to paper is maintained for the test. It is more important to have two readings to average than one test with the full gram of paper. I would like to thank Mr. Wilson of the National Archives and Mr. Bohanan of TAPPI for discussing this matter with me. 88. Barbara Miller, personal communication, 4/83; see also articles such as the study by Graminski, Parks and Toth in Restaurator, 2, 1978, pp. 175–178, where it is recorded that little if any degradation of their specimens occurred at 0% RH regardless of the temperature setting of the oven (p. 176). The scientists who deal with consumer questions at Customer Services of the Sigma Chemical Company, M. Mahinka of the Novo Laboratories, and Dr. M.D. Appleman, formerly of the University of Southern California, do not believe that there is any chemical reason that an enzyme should discolor or leave a colored material in paper. An article in Technology (see note 4) also speaks of enzymes as “colorless compounds”.Apparently, if an enzyme is pure enough, it should not discolor. In a personal communication with Victoria Blyth Hill of the Los Angeles County Museum of Art, the author was made aware of tests ran by Leslie Kruth while working at the museum in 1983. She found that Whatman papers yellowed with oven aging if they were dipped into solutions of trypsin and α amylase and dried without rinsing. Rinsed samples did not yellow. Trypsin is not the purest of preparations, however, and this might explain at least one of the results obtained. 89. The mean and standard deviation were calculated on Hewitt Packard HP45 calculator, which uses the formula
90. Dr. McComb and Dr. Tang, Preservation Office, Library of Congress, personal communication, 4/83. 91. Indeed, using the “student t” test for probability, the only comparisons which fell below eighty repeatable experiments out or 100 for preaged specimens are comparisons between specimen groups 3 and 4, and 1 and 5. For post-aged
92. See, for example, J. Nelson, A. King, N. Indictor, D. Cabelli, “Effects of Wash Water Quality on the Physical Properties of Three Papers”, Journal of the American Institute of Conservation, v. 21, no. 2, Spring 1982; Wilson, Golding, McClaren and Gear, “The Effect of Magnesium bicarbonte Solutions on Various Papers” pp. 87ff., in J. Williams, ed. Preservation of Paper and Textiles of Historic and Artistic Value II, Advances in Chemistry Series #193, American Chemical Society, Wash., D.C., 1981; L. Tang and N. Jones, “The Effects of Wash Water Quality on the Aging Characteristics of Paper”, Journal of the American Institute of Conservation, 1979, 18(2), 61–81. 93. This result could be due to the hour long soak in warm water followed by another hour long immersion, and not due to any effect of the enzyme per se. 94. This result could be due to the difference in pH: deizonized water, 5; enzyme solution, 6. It is interesting that the paper retained the pH of the enzyme solution. 95. M. A. Johnson has stated that both conditions could be moderately non-optimal and one could still achieve good results (personal communication, 10/8/83). 96. A ten minute bath in deionized water raised to pH 7.8 with calcium and magnesium inactivated the enzyme in primitive tests devised by the author (as described above). To determine whether or not the enzyme was irreversibly inactivated would require reagents and equipment not available to her at this time. |