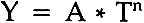THE EFFECT OF AN ALKALINE RINSE ON THE AGING OF CELLULOSIC TEXTILES, PARTS I AND IIIra Block
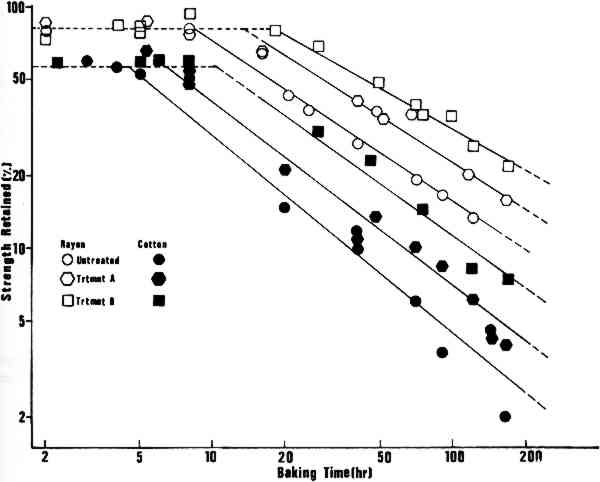
1 PART I: PRELIMINARY RESULTS1.1 INTRODUCTIONDURING A SABBATICAL YEAR tenure at the Conservation Analytical Laboratory of the-Smithsonian Institution, the author studied the efficacy of the deacidification of cellulosic textile materials as a conservation treatment. In this series of papers the some-what unexpected results of this investigation are reported. In Part I, the reasons for believing that deacidification would be effective are discussed, and preliminary results on new rayon and cotton cloth are presented. Part II reports a detailed examination of the kinetics of strength loss and color change. Part III will report the effect of a calcium hydroxide rinse on artifically aged cellulosics, which turns out to be less effective than on new cloth. This leads to the prediction that an alkaline rinse will not be more effective than soaking in deionized water when treating old cellulosic fabrics. Part IV shows that the conclusions reached in Part III are valid and explains why. It is shown that provision of an alkaline reserve is effective in retarding the degradation of new fabrics, but is useful for old fabrics only in protecting against the absorption of acidic species from the environment. In a recent review article, Daniels1 pointed out that as early as 1898 it was known that the acidic impurities in paper could greatly increase its rate of deterioration. Shaw and O'Leary2 noted in their investigations on the stability of book papers that “calcium carbonate pigments had a protective or inhibiting effect in the aging test,” and Barrow's3 investigation of the causes of deterioration of books showed that treatment with an alkaline solution could increase the life of books by neutralizing the acids present in the paper and by providing an alkaline reservoir which would shield against further acidification. Although it is well known that washing or rinsing old paper in clean, pure water can improve its color, strength and folding endurance, it has been only recently that the alkaline treatment of nearly neutral papers has been investigated. Tang and Jones4 noted that “Distilled or deionized water passed through a column of calcium carbonate chips becomes acceptable for paper washing, and can be used without shortening the life of the paper.” Wilson, et al5 have discussed the efficacy of magnesium bicarbonate treatment of old papers, and other workers have investigated the protection afforded paper when rinsed with amines6, 7 and organometallic compounds.8, 9 The above work is of some significance to the conservation of textile products, since cellulosic fibers such as cotton, linen, jute and rayon comprise an appreciable fraction of textile artifacts. These materials are often washed as part of their conservation treatment, and the usual procedure for the care of textiles,10–15 as was once the case for the treatment of paper,16 has been to use water of as high a quality as possible. However, if when washing is indicated, a mildly alkaline rinse could enhance the resistance of cellulosic textile fibers to degradation at ambient temperatures, conservators would be provided with an important new tool for treating many fabrics. The literature indicates that it should be possible to stabilize cellulosic textiles, for which washing is indicated as part of the conservation treatment, by a mild alkaline rinse which would neutralize the internal acids. This procedure appears to have been originally recommended by Pahir in a 1925 patent and rediscovered by Wilkie in 1929.17 In addition to the literature on paper conservation, other work leads to this conclusion. Peters and Still18 point out that it “is difficult to differentiate the thermal degradation at low temperatures from the normal aging of cellulose.” They note that the degradation of cotton is greater in air than in nitrogen and is enhanced by moisture, and suggest that the formation of carboxyl (− COOH) and carbonyl (>C = 0) groups is important in the degradation of cellulose. Arney, et al19, 20 argue that the degradation of cellulose in paper is due to both oxygen-dependent and oxygen-independent processes, and that both types of processes are affected by deacidification treatments. They further state that their results suggest that “the influence of acidity on the rate of natural paper degradation would be the same regardless of which degradation process predominates at room temperature.” In a series of investigations, Davidson and Nevelle21 studied the degradation of acidic oxycelluloses. They were able to show that a major cause of the degradation of cellulose is the formation of carboxyl groups on the C6 carbon of the anhydroglucose unit. Davidson and Standing22 hypothesized that “hydrolysis of the glycosidic linkages is catalysed by hydrogen ions derived from the ionization of the free (unneutralized) carboxyl groups in the oxycellulose molecule.” It was shown that oxycelluloses containing the free acid were unstable even when stored at room temperature and low relative humidities, and that conversion of the carboxyl groups from the free acid to the sodium salt form greatly stabilized the cellulose.23 More recent studies by Shenai and Shah24 among others, supported these conclusions. 1.2 EXPERIMENTALTHE ACCELERATED AGING of fibrous materials by oxidation in both dry and humid ovens at various temperatures, with and without light, has been intensively studied.25–38 Since artificially degraded fabrics do not duplicate naturally aged materials in all respects (cf reference 39), it is necessary to choose a test protocol in which the chemical processes do not differ substantially from those occurring at room temperature, yet proceed rapidly enough to allow results to be obtained in a reasonable time period. As noted earlier,18 low temperature thermal degradation is almost identical to normal aging. The problem is to determine how high a temperature can be considered “low”. Wilson and Parks25 noted that artificial aging at 100�C “with some moisture” gave results that were closer to naturally aged paper than did artificial aging at 90�C and 50% R.H. Unfortunately, cellulosic textiles do not age rapidly enough at 100�C to permit a reasonable time frame for the experiment. In this work it was found that dry baking at 100�C for one week caused essentially no change in fabric properties, in It should be noted that at this writing the only accepted standards for aging of textile materials are those promulgated by the American Association of Textile Chemists and Colorists (AATCC). These tests, AATCC 26-1978 and 111A,B,C,D-1978, are specifically devised for determining the resistance of textiles to sulfur dyes and weather resistance, respectively, and are not applicable to comparisons of the relative effectiveness of methods for reducing the rate of degradation of fabrics. Thus, the author has endeavored to apply a test method which would not introduce extraneous chemical reactions and which would permit comparisons between treatments. Although it is recognized that other environmental factors such as light, relative humidity and atmospheric gases will affect the results of both natural and accelerated aging, it was decided to limit this study of the effect of elevated temperature and low humidity. In this way, different treatments can be compared with a minimum of extraneous factors, while still taking into account the important role played by moisture. 1.2.1 FabricThe fabrics in this study were a plain weave, 80 � 80 cotton print cloth weighing 103 g/sq m (no. 400) and a plain weave, 80 � 80 spun rayon cloth weighing 132 g/sq m (no. 266) supplied by Testfabrics Inc. The cloths were washed and dried twice as per AATCC Test Method No. 124-1978. 1.2.2 ReagentsThe reagents used were calcium hydroxide [Ca(OH)2] and sodium carbonate [Na2CO3] supplied by Fisher Scientific Co., and deionized water of about 1 megohm resistance. Calcium hydroxide solution was prepared by adding at least three grams of solid to one liter of water, shaking and allowing the solids to settle overnight. Fifty milliliters of the saturated solution were made up to one liter to provide an approximately 0.01% (w/v) solution with a pH of 9–9.5. A saturated solution of sodium carbonate was prepared in a similar manner, and 1.4 ml were made up to one liter to give an approximately 0.01% (w/v) solution. 1.2.3 ApplicationThe test fabrics were cut to the proper dimensions with an NAEF die. Nine of the specimens were added to a one-liter bath of solution maintained at 30�C by circulation of the solution through a coil of polyethylene tubing immersed in a temperature-controlled bath. Solution pH was maintained by dropwise addition of the saturated solution [(Ca(OH)2 or Na2CO3 as appropriate] to the rinse bath. For treatment A, the specimens were soaked for 2 hr in Ca(OH)2 solution maintained at a pH between 9.0 and 9.5, removed from the bath, and soaked an additional hour in the Na2CO3 solution at 30�C to precipitate CaCO3,19 drained and dried overnight on glass fiber screens. For treatment B, specimens were soaked for 3 hr in Ca(OH)2, removed from the bath, given a fast rinse in deionized water to remove surface solution, drained and 1.2.4 AgingEach sample was made up of three specimens of fabric chosen at random from the treated or untreated materials as applicable. Each of the specimens in a sample was supported on a glass fiber screen while being baked in a Fisher Isotemp forced draft oven at 150 � 2�C. Since the oven was open to the atmosphere in the laboratory (approx. 21�C and 55% RH) the relative humidity in the oven varied, but remained low, in keeping with the recommendation of Wilson and Parks25 and the work of Williams, et al.27 Upon removal from the oven after baking, samples were stored in a closed container over silica gel until cool. After cooling samples were stored in sealed plastic bags until testing. 1.2.5 Tear TestingPrior to testing, the bags containing the samples were opened and the samples were stored at standard conditions of 21�C and 65% RH for at least twelve hours. The degradation of the samples was measured by the loss in tear strength. This parameter was chosen since, as the work of Berry, et al.39 shows, both tear strength and tensile strength follow essentially the same kinetics; tear testing is more rapidly performed than tensile testing; and tearing more closely approximates the stresses put on a fabric when handled than does tensile testing. Tear testing was performed on an Elmendorf Apparatus as per ASTM D 1424–63, at 21% and 65% RH. 1.3 RESULTS AND DISCUSSIONIN A SERIES OF preliminary experiments it was found that soaking in Ca(OH)2 solution for 2 hours gave the same weight gain as soaking for five hours. Thus, the 2-hr or the 3-hr soak provided the maximum uptake of calcium, about 2% calcium carbonate on weight of fabric (owf). The surface pH of the samples was measured with an lonalyzer fitted with a flat-bottomed electrode. It was found that the untreated fabric samples had a pH of 5.5 to 6.0, that the pH of samples immediately after soaking was between 8.0 and 8.5, and that the pH fell to between 7.0 and 7.5 after drying overnight. Tear strength and color of the treated samples was measured and found to be essentially the same as the control fabric. Subjective evaluation of the hand indicated that the treatments did not cause harshening or brittleness. The major results of this portion of the study are shown in Fig. 1. The logarithm of the percent strength retained is plotted versus the logarithm of the baking time. This corresponds to an equation of the form
The curves of Fig. 1 reveal three important features: (1) an initial period in which log(Strength Retained), (Y), is insensitive to the log(Baking Time), (T), (2) a later period in which Y decreases linearly with T, and (3) the point at which Y becomes sensitive to T. Since the early data for both treated and untreated fabrics fall on a single line with zero slope, we may conclude that identical processes are at work in the cotton samples irrespective of treatment, and that the rayon samples are subject to the same processes irrespective of treatment. However, the chemistry of the rayon system may be different from that of the cotton. At some point, Y begins to decrease linearly with T. The slopes of the lines are: Regression analysis indicates that there is no significant difference between the slopes of the three lines for each of the respective fibers. Thus we may, again, conclude that identical processes are operating in the cotton samples irrespective of treatment, and that the same holds true for the rayon cloth. The time at which the alkaline treatment begins to lose its efficacy, (Tc), is found to be about 4.5 hr for the untreated cotton, about 7 hr for the samples subjected to Treatment A and about 10 hr for the samples which were given Treatment B. For the rayon samples, these events occur at about 8 hr, 12 hr and 19 hr, respectively. It is interesting to note that in both cases the ratio of Tc for Treatment A to Tc for the control is about 1.5 and the ratio of Tc for Treatment B to Tc for the control is about 2.5. This equivalence indicates that the treatment is effective irrespective of the source of the cellulose, and may mean that the chemistry of the degradation process is independent of the source of the cellulose. Treatment A is not as effective as Treatment B, probably because rinsing did not remove all of the sodium ions. Clapp has pointed out the deleterious effects of sodium on the aging of paper.16 To test this hypothesis a set of samples was subjected to Treatment A, but given only a fast rinse in deionized water. The treated samples degraded faster than the controls. We may postulate, then, that the effect of the calcium carbonate is to inhibit the deterioration processes, and that at some point the calcium ions are no longer effective. It is at this point that the slope of Y vs T abruptly changes. 1.4 CONCLUSIONSFROM THE RESULTS to date, it appears that soaking new cotton or rayon cloth for about 3 hours in a 0.01% calcium hydroxide bath at 30�C and allowing it to air dry can increase its lifetime by about a factor of 2.5 without affecting color or hand. |