A TECHNICAL NOTE ON NIELLOR. Newman, J. R. Dennis, & E. Farrell
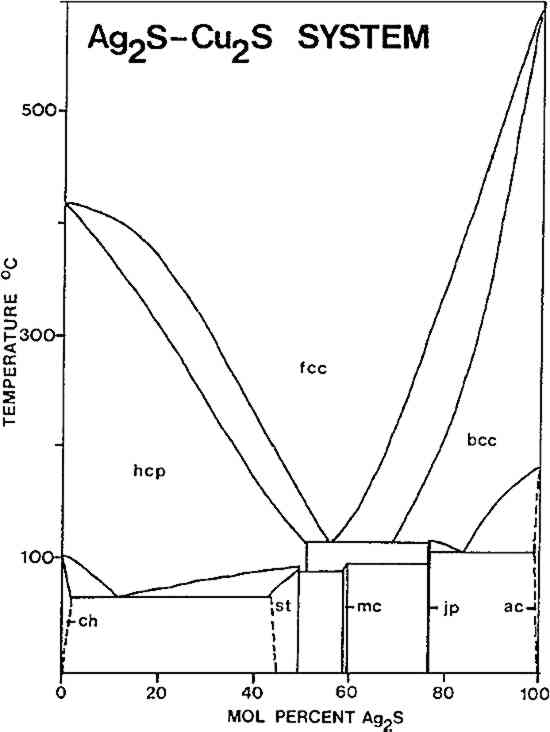
3 PROPERTIES OF NIELLORELATIVELY PURE COPPER sulfide (equivalent to the mineral chalcocite, Cu2S) or silver sulfide (acanthite, Ag2S) niello must be applied by grinding up the material, packing it into the area to be decorated, and heating and burnishing repeatedly to fix it into place. Both sulfides are malleable at room temperature, and much more so when heated, but both decompose in air before melting, and thus they cannot be fused into place. On the other hand, silver-copper-lead niello is readily fusible, and thus may be applied by this more rapid method. The lowest melting mixture in this system melts TABLE II NIELLO COMPOSITIONS FROM 6TH-CENTURY BYZANTINE OBJECTS Samples arranged by approximate Ag/Cu contents (estimated by emission spectrography) Niello consisting only of silver-copper sulfide is also potentially fusible, but only at temperatures of some 200�C higher than the melting point of the “eutectic” silver-copper-lead sulfide composition. There are five phases in the Ag2S-Cu2S system at 25�C16 Skinner found that the stable assemblage at room temperature will be one or more of these phases.17 The phase diagram of the system is reproduced in Figure 1. The fcc solid solution shown in the diagram is effectively unquenchable. The minimum of the liquidus in this system (the minimum melting temperature) is about
The silver-copper niello of Pliny's recipe contains substantially more copper than any of the ones we have examined, and would actually be a somewhat lower-melting composition than our samples. Similarly, the two silver-copper nielli reported by Schweizer are also lower-melting ones (one has a Cu:Ag ratio of about 2:1 and the other a Cu:Ag ratio of nearly 1:4).19 In our study, two samples of niello were taken from different areas of three of the objects examined. In only one instance were the two samples similar or identical (DO 3 and DO 4). In the other two cases, one was a nonfusible Ag2S type and the other a potentially fusible silver-copper sulfide type. Whether one was fused into place and the other burnished, or whether both were burnished, is a matter of conjecture, although it may be the case that copper has been depleted in some of the sampled areas more than in others, and the present composition of the niello does not accurately reflect the original composition. Oddy et al. have also found an object with a silver-copper niello in one place and a silver niello in another place.20 In visual appearance, none of the various types of niello which have been described above differ appreciably from one another. The most important differences are in handling properties as they are applied to objects. Analyses of niello are more numerous from the Byzantine period than any other time, and these analyses show that both fusible and nonfusible types of niello were apparently already in use by early Byzantine craftsmen. ACKNOWLEDGEMENTSThe authors wish to thank Dumbarton Oaks for allowing us to sample objects from their collection for this study. The scanning electron microscope work was carried out at the Center for Astrophysics, Harvard University-Smithsonian Institution, Cambridge, Massachusetts. We thank Dr. J. A. Wood and Karen Motylewski for allowing us to use this instrument. |