A TECHNICAL NOTE ON NIELLOR. Newman, J. R. Dennis, & E. Farrell
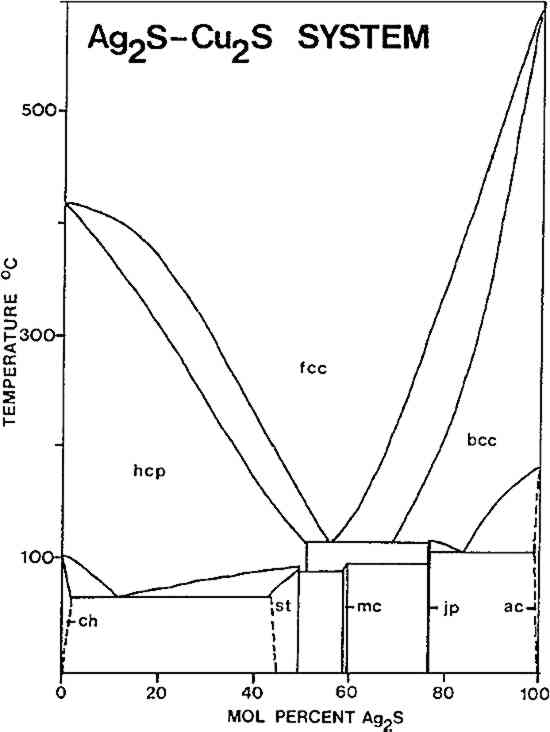
ABSTRACT—Niello, a lustrous blue-black material, consists of one or more metallic sulfides (silver, copper, and lead). It is used to decorate silver, bronze, and gold objects. The history of the use of niello is reviewed, as well as the properties of the various types. 1 INTRODUCTIONNIELLO IS A LUSTROUS blue-black substance used to enrich and heighten engraved and chased ornaments and inscriptions on silver, bronze, and gold objects. More rarely, it may have been used as a kind of “ink” on nonengraved surfaces.1 Niello consists of one or more metallic sulfides (sliver, copper, and lead), and, depending upon its precise composition, may either be fused or burnished into place. Surviving classical, medieval, and Renaissance recipes indicate that virtually every possible combination of the three types of sulfides may have been used as niello, although not all of these variations have been identified on objects to date. In some instances, niello could conceivably be confused with other black substances on metal objects: chemical patinas on copper alloys; lacquers or dark resinous materials; enamel; and so forth. All of these may be readily distinguished from niello by analytical tests. This note summarizes the history of niello and some of the properties of the various types. For the sake of brevity, various types will be referred to by the metals which have entered into their compositions (e.g., “copper niello” for copper sulfide, etc.) 2 HISTORY OF NIELLOTHE NUMBER OF published analyses of niello remain few; consequently the history of the use of the different types is still not well known. A limited number of analyses were published by Moss in 1953;2 Schweizer has reported analyses of Byzantine niello;3 and Oddy et al. have recently published 18 new analyses.4 The analyses of 12 niello samples from nine mid-6th century Byzantine silver objects in the Research Library and Collection, Dumbarton Oaks, Washington, D. C., are reported in this paper.5 Copper niello has thus far been identified only on Roman objects (1st–4th century).6 Published analyses suggest that silver niello was used earlier than silver-copper or silver-copper-lead niello: silver niello has been identified on Roman objects from as early as the 2nd century,7 while mixed types have not been found on objects of earlier than medieval dates. However, classical and medieval treatises indicate that these mixed types were known as early as the silver niello. Pliny gives a recipe for a silver-copper niello,8 and the medieval treatise, the Mappae Clavicula, contains a recipe for silver-copper-lead niello.9 A summary of several recipes is given in Table I with the relative proportions of sulfides in each. The method of preparation normally involves mixing simple proportions of metals together and burning these with excess sulfur. TABLE I COMPOSITIONS OF NIELLO FROM VARIOUS TREATISES The earliest analyzed examples of silver-copper niello are of 6th-century dates. Oddy et al. found such a niello on a gilded silver Anglo-Saxon brooch,10 while Schweizer found such a niello on a 6th–7th century Byzantine silver object.11 Of the 12 samples we have studied from 6th-century Byzantine objects, six are silver-copper niello, with copper contents (estimated by emission spectrography) in the ranges 1–10% (one sample) and 10–15%(five samples). The remaining six samples were either relatively pure silver sulfide or silver sulfide with less than 1% copper. A summary of these 12 samples is given in Table II. Silver-copper-lead niello has not been identified on objects of early medieval date. Schweizer found one such niello on an 11th–12th century Byzantine object, 12 while Oddy et al. found a sample on a 13th-century object.13 3 PROPERTIES OF NIELLORELATIVELY PURE COPPER sulfide (equivalent to the mineral chalcocite, Cu2S) or silver sulfide (acanthite, Ag2S) niello must be applied by grinding up the material, packing it into the area to be decorated, and heating and burnishing repeatedly to fix it into place. Both sulfides are malleable at room temperature, and much more so when heated, but both decompose in air before melting, and thus they cannot be fused into place. On the other hand, silver-copper-lead niello is readily fusible, and thus may be applied by this more rapid method. The lowest melting mixture in this system melts TABLE II NIELLO COMPOSITIONS FROM 6TH-CENTURY BYZANTINE OBJECTS Samples arranged by approximate Ag/Cu contents (estimated by emission spectrography) Niello consisting only of silver-copper sulfide is also potentially fusible, but only at temperatures of some 200�C higher than the melting point of the “eutectic” silver-copper-lead sulfide composition. There are five phases in the Ag2S-Cu2S system at 25�C16 Skinner found that the stable assemblage at room temperature will be one or more of these phases.17 The phase diagram of the system is reproduced in Figure 1. The fcc solid solution shown in the diagram is effectively unquenchable. The minimum of the liquidus in this system (the minimum melting temperature) is about
The silver-copper niello of Pliny's recipe contains substantially more copper than any of the ones we have examined, and would actually be a somewhat lower-melting composition than our samples. Similarly, the two silver-copper nielli reported by Schweizer are also lower-melting ones (one has a Cu:Ag ratio of about 2:1 and the other a Cu:Ag ratio of nearly 1:4).19 In our study, two samples of niello were taken from different areas of three of the objects examined. In only one instance were the two samples similar or identical (DO 3 and DO 4). In the other two cases, one was a nonfusible Ag2S type and the other a potentially fusible silver-copper sulfide type. Whether one was fused into place and the other burnished, or whether both were burnished, is a matter of conjecture, although it may be the case that copper has been depleted in some of the sampled areas more than in others, and the present composition of the niello does not accurately reflect the original composition. Oddy et al. have also found an object with a silver-copper niello in one place and a silver niello in another place.20 In visual appearance, none of the various types of niello which have been described above differ appreciably from one another. The most important differences are in handling properties as they are applied to objects. Analyses of niello are more numerous from the Byzantine period than any other time, and these analyses show that both fusible and nonfusible types of niello were apparently already in use by early Byzantine craftsmen. ACKNOWLEDGEMENTSThe authors wish to thank Dumbarton Oaks for allowing us to sample objects from their collection for this study. The scanning electron microscope work was carried out at the Center for Astrophysics, Harvard University-Smithsonian Institution, Cambridge, Massachusetts. We thank Dr. J. A. Wood and Karen Motylewski for allowing us to use this instrument. REFERENCESThree of the six niello recipes in the Mappae Clavicula, a manuscript probably of 9th-century origin, direct that the metallic sulfides be ground up, mixed with vinegar to form an ink, designs written with this ink on the vessel, and the designs then heated to fix them into place. See recipes 56, 89b, and 206 in: C.S.Smith and J.G.Hawthorne, “Mappae Clavicula. A Little Key to the World of Medieval Techniques,” Transactions Am. Phil. Society, new series, 64 (1974). Moss, A.A. “Niello.” St. in Cons.1 (1953), pp. 49–61. Schweizer, F., in “Objets byzantine de la collection du Mus�e d'art et d'histoire.” Genava, new series, 25 (1977), pp. 51–61. Oddy, W.A., S.LaNiece, and M.Bimson. “Composition of Niello Decoration on Gold, Silver, and Bronze Objects in the Antique and Medieval Periods,” Submitted to St. in Cons. The results of these analyses were previously described in: J.R.Dennis, “Niello: A Technical Study.” Papers presented by trainees at the Art Conservation Training Programs Conference, April 30, May 1 & 2, 1979, Center for Conservation and Technical Studies, Fogg Art Museum, Harvard University, pp. 83–95. Reference 4, analyses 1–3. Reference 2, p. 61. Pliny Hist. nat. XXXIII, Ch. 46. Reference 1, recipe 56. Reference 4, analysis 10. Reference 3, p. 59. Ibid. Reference 4, analysis 18. Schwarz, R. and A.Romero. “Zur Kenntnis der Tulalegierung (Eine Untersuchung uber das Dreistoffsystem Cu2S-Ag2S-PbS).” Zeitschrift fur anorganische u. allgemeine Chemie162 (1927), pp. 149–160. Amcoff, O. “The solubility of silver and antimony in galena.” Neues Jahrb. Min., Mh.,1976, pp. 247–261. Phases in this system have been studied by: (a) Djurle, S. “An X-ray study on the system Ag-Cu-S.” Acta Chem. Scandinavica12 (1958), pp. 1427–1436. (b) Skinner, B.J. “The System Cu-Ag-S.” Eco. Geo.61 (1966), pp. 1–26. Reference 15(b), p. 10. Grybeck, D. and J.J.Finney. “New Occurences and Data for Jalpaite.” Am. Mineralogist53 (1968), pp. 1530–1542. Reference 3, p. 59. Reference 4, analysis 17.
 Section Index Section Index |