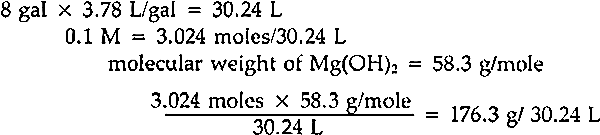NOTES ON A PRESSURIZED SYSTEM FOR PRODUCING MAGNESIUM BICARBONATE SOLUTIONSRandall Couch
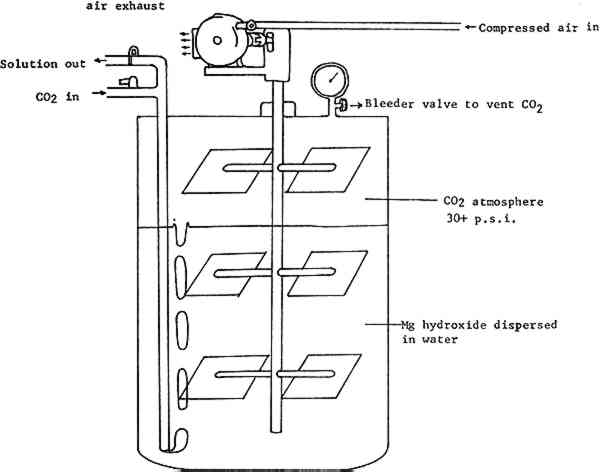
ABSTRACT—Magnesium bicarbonate is frequently used for the deacidification of paper artifacts. The author describes a pressurized system for producing magnesium bicarbonate solutions and discusses some of the major factors affecting its performance. Practical tests indicate that 1) the system's rate of production could be increased by improved dispersal of carbon dioxide gas and 2) the apparatus is not capable of generating a clear solution even under optimum conditions. The system is modified by the addition of a settling tank. Suggestions are made for further investigation in the design of new pressurized systems. 1 INTRODUCTIONAN EFFICIENT SYSTEM for producing aqueous deacidification solutions is necessary in any large paper conservation laboratory. Such a system should consistently generate a large volume of solution with good working properties in a reasonable period of time, and should be sparing in its use of raw materials and staff time. The apparatus for producing magnesium bicarbonate at Northeast Document Conservation Center was recently evaluated to determine whether improvements could be made in any of these areas. The tests performed were simple and based on well-known theoretical work. Because NEDCC is one of only a few institutions using a pressurized system for making magnesium bicarbonate solutions, our observations may be of interest to others. It is hoped they will be useful in more systematic research on pressurized systems. 2 SOLUTION PROPERTIESTHE PROPERTIES OF DEACIDIFICATION solution relevant to this evaluation are clarity and concentration. Clarity, or freedom from undissolved and extraneous solid matter, is usually measured by visual inspection, what is “clear enough” being a matter of judgment. Concentration can be tested by several less subjective methods. The optimum concentration for a given application is, however, controversial, relating to the amount of alkali left in the treated paper. Santucci1 noted that a two-percent alkaline reserve, based on studies of well-preserved early volumes, was proposed as early as the 1930s. Both the National Archives2 and the Library of Congress3 have investigated the relationship between concentration of deacidification solutions and alkaline reserve. The Library of Congress recommends a 2–3% deposit of alkali, or 400–600 milli-equivalents in a kilogram of paper. National Archives tests show that a magnesium bicarbonate solution of about 0.1 M concentration is required to produce this reserve in a normal dipping treatment. TheTaylor Hardness test (titration for dissolved magnesium with EDTA) is a convenient test of concentration, which indicates a 0.1 M solution by a minimum of 18 ml of titrant.4 While a more dilute solution may be appropriate in many cases, the capability of generating a water-clear solution of this strength was chosen as a standard for evaluating our system's performance. At the time we began testing, our solutions were normally neither clear nor of optimum concentration. 3 SYSTEMTHE SYSTEM CENTERS AROUND a modified paint-mixer made by the Binks Mfg. Co. (Fig. 1). A rotary stirrer provides horizontal and vertical agitation of the solution. The stirrer is powered by compressed air from an outside compressor. Carbon dioxide under pressure enters through a straight pipe and bubbles up through the soliution from the bottom. Eight gallons of deacidification solution are made at a time, with a carbon dioxide atmosphere filling the rest of the chamber's ten-gallon capacity. All interior surfaces of the chamber and stirrer are stainless steel.
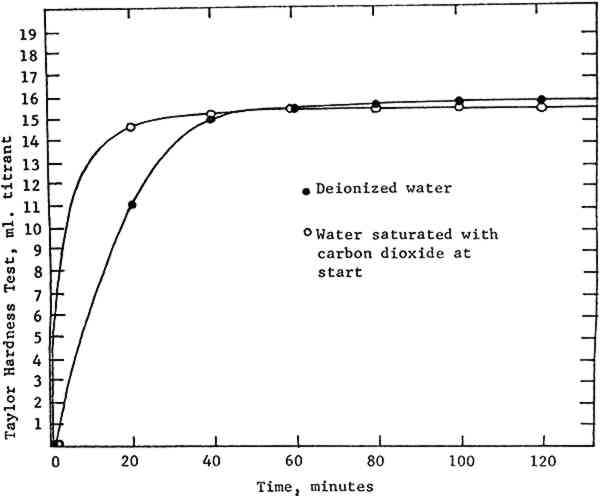
The equipment was originally used with magnesium carbonate powder as a starting material. Since the publication in 1978 of National Archives test results showing magnesium hydroxide to have a substantially higher rate of solubility, that material has been employed. The magnesium hydroxide powder is first passed through a wire strainer to break up any lumps and then mixed with water in a blender for several minutes to disperse it further. This mixture is poured into the mixer and water is added to reach the eight-gallon mark. The chamber is capped, the stirrer and CO2 turned on, and the reaction allowed to proceed for the requisite period. The finished solution is pumped out using only the mixer's internal pressure. 4 MAGNESIUM HYDROXIDEWE FIRST EXAMINED THE EFFECT of variations in the quantity and quality of our starting material. The concentration of magnesium bicarbonate solutions varies proportionally with the amount of magnesium hydroxide used. In an eight-gallon volume, a minimum of 176.3 grams of magnesium hydroxide is necessary to produce a 0.1 M solution.5 A solution of this concentration was not regularly employed at NEDCC because solutions of lower concentration were often too cloudy for immediate use. Reasons for this cloudiness were investigated. Although about one percent of magnesium hydroxide powder is expected to be insoluble due to impurities,6 it was not certain that the observed cloudiness could be attributed to this factor alone. Another possible cause of cloudiness was the age of our magnesium hydroxide supply. Since this material tends to convert to the basic carbonate on exposure to air, some contamination could occur under practical storage conditions. If the slower-dissolving carbonate was present in any quantity, a cloudy solution would result. A fresh shipment of magnesium hydroxide, however, made no appreciable difference in clarity. Solutions made from it remained cloudy and no change in concentration was noted. Therefore we concluded that no practical improvement could be made based on the quality of the magnesium hydroxide. 5 WATERTHE WATER SUPPLY AT NEDCC is filtered to remove particulates. It contains some alkalinity (calcium and magnesium compounds) and has a pH which averages 8–9.7 Since magnesium hydroxide is insoluble in basic solutions, it seemed possible that using tap water could slow the dissolving of the powder, and account for the persistent cloudiness. The use of deionized water,8 however, gave only minor improvements in rate, and no observable improvement in clarity of the solution. 6 CARBON DIOXIDEMAGNESIUM BICARBONATE is formed from magnesium hydroxide and carbon dioxide according to the reaction Mg(OH)2+2CO2 → Mg(HCO3)2. If this reaction occurs under pressure, the concentration of dissolved carbon dioxide is increased, and Le Chatelier's principle requires that the concentration of product must also increase to restore equilibrium. This is the advantage of pressurized systems for producing deacidification solutions. The surface area of contact between reactants is also important, since the reaction cannot take place except where they meet. The surface area of powder can be maximized by straining and dispersing it in the blender as we have done. The surface area of CO2 is maximized by bubbling, and in this respect our system is not very efficient. There are two reasons for this. The first cause is inherent in the closed design of the system. When CO2 gas enters the mixing chamber, it bubbles up through the liquid to form an atmosphere which quickly (in a matter of minutes) reaches a pressure equilibrium with the gas source. In our system this happens when the chamber pressure reaches roughly 32 p.s.i. The result is that bubbling comes to a halt—gas can only enter as fast as it is consumed in 1) saturating the water and 2) the bicarbonate reaction itself. After the gas stops bubbling up through the liquid, the site of the reaction is soon limited primarily to the surface of the water, which is agitated only moderately by the stirrer. There is a partial solution to this problem. The chamber is equipped with a screw-type bleeder valve, which can be adjusted to vent the CO2 atmosphere at a The second factor affecting CO2 dispersion is bubble size. Gas enters our mixing chamber through a straight pipe with an inside diameter of ⅜ inch. If the same volume of gas were forced through the numerous 10—15 micro-meter openings of a diffuser, an increase in CO2 surface area of several orders of magnitude would result. Additionally, the small bubbles would take longer to rise to the surface, thus prolonging the time of contact between liquid and gas bubbles. A diffuser offered an obvious opportunity to gain efficiency in the system. However, it would require expensive alterations to the mixer, since adding a diffuser would prevent the same tube from being used to pump out the finished solution. Before having another tube welded and sealed into the mixer, a test was devised to determine what sort of benefits would result from better dispersion of CO2. Two quantities of deacidification solution were produced: one in the traditional manner with deionized water, and a second in which the water was saturated with CO29 at the start. Both quantities were subjected to bubbling and pressure as described above. Samples were drawn every 20 minutes and titrated using the Taylor test. Figure 2 summarizes the results.
As anticipated, the initial reaction rate was significantly faster using carbonated water. However, the “plateau of solubility” or maximum obtainable concentration was roughly the same in both trials. Carbonated water produced a bicarbonate solution of slightly greater clarity than did deionized water, but in both cases some turbidity remained after two hours of bubbling. Improved CO2 dispersion, then, could shorten the necessary bubbling time, but by itself could not accomplish the task of generating a clear solution of sufficient strength. Modification of our present equipment to accommodate a diffuser was thus judged to be not worthwhile, though such a device should be considered in the design of new systems. 7 TEMPERATUREDISSOLUTION OF CARBON DIOXIDE is promoted by low temperatures. Where chilled water systems are available, they should be employed in making magnesium bicarbonate solutions. NEDCC does not have such a water supply, and no significant seasonal variations in rate were noted; it is possible that such variations may have been obscured by manipulation of other factors. 8 CONCLUSIONDESPITE THE ADVANTAGES in rate provided by a pressurized system, it was not possible with our equipment to make a magnesium bicarbonate solution clear enough for immediate use, even at concentrations lower than our target of 0.1 M. The persistent cloudiness resulted, perhaps, from some process such as the formation of magnesium carbonate within the system. It was therefore necessary to add an intermediate step—a settling tank—between the preparation of the solution and its use on paper objects. Settling is common to most bulk methods of producing deacidification solutions, and those which start with an excess of magnesium carbonate generate large quantities of sediment. The amount produced by our solutions is much smaller. We chose a 25-gallon polyethylene settling tank, so that, when demand warrants, three batches of solution can be produced in succession and allowed to settle at the same time. Using 180 grams of magnesium hydroxide powder (slightly more than required) each batch reaches the desired concentration of 0.1 M in ninety minutes. The settling time necessary to eliminate cloudiness varies; two to four hours is typical. The clear solution is then drawn from the tank's spigot, located above the sediment line, and transferred to polyethylene jerry-jugs for storage and use. Exhaustive studies of the deterioration of magnesium bicarbonate solutions over time are not available. National Archives data10 suggest that solutions in the 0.1 range decrease in concentration less than 1% per day over the first four days. This decrease is usually accompanied by precipitation at the solution-air interface and the solution-solid interface (if the solution stands in contact with the solid from which it is made). Our observation in practice is that surface precipitation first occurs from two to five days after the solution is made. When precipitation is recognized, the solution is thrown out—a rare occurrence at NEDCC due to the volume used. A second titration of the clarified solution is performed when its concentration is in doubt. Storage in jerry-jugs is consistent with the principle that magnesium bicarbonate solutions should not stand in contact with the solids from which they are made, or with large volumes of air. It has the additional advantage of avoiding the deposition of metal ions which can occur in stainless steel tanks. The addition of a settling tank was the only modification to our equipment judged cost-effective. For the design of new pressurized systems, however, the following areas might be profitably investigated:
ACKNOWLEDGEMENTSMany people at the Northeast Document Conservation Center contributed suggestions to this project, particularly Sherelyn Ogden, for whose advice and support I am very grateful. REFERENCESSantucci, L. “Paper Deacidification Procedures and Their Effects.” Colloques Internationaux du CNRS 548 sur Les techniques de Laboratoire dans l'Edude des Manuscripts (September 1972), p. 198. Wilson, W.K., et al.Preparation of Solutions of Magnesium Bicarbonate for Deacidification of Documents. Washington, D.C.: National Archives of the United States, 1979. “The Deacidification and Alkalization of Documents with Magnesium Bicarbonate.” Library of Congress Publications on Conservation of Library Materals, Conservation Workshop Notes on Evolving Procedures. Series 500: No. 1., Working Draft, July, 1978. The procedure for performing the Taylor Hardness test is given in the Library of Congress Workshop Note cited above. All titrations discussed in this paper were done in conformity with this procedure. Note especially that all samples are filtered before titration, as the presence of undissolved magnesium compounds can invalidate the results. The Taylor Total Hardness Set, catalog no. 1123, includes all necessary chemicals and some equipment, and is available from Taylor Chemicals, Inc., 7300 York Road, Baltimore, MD 21204.
Wilson, W.K., et al. “Preparation of Solutions of Magnesium Bicarbonate for Deacidification.” The American Archivist, vol. 41,No. 1 (January 1978), p. 68. Filter type: Culligan HD-20, backwashable, filters particulates to 10 microns. Mineral analysis at source provided by Town of Andover, July 1980. Deionizer: a mixed-bed resin supplied by MWM Company, Inc., 11 Newbury St., Quincy, MA 02171. Order description IRN150. For the limited purposes of this test, the most convenient source of carbonated water was deemed satisfactory. Canada Dry Salt-Free Seltzer, unlike ordinary “tasty” seltzers and club soda, lists carbonated water as its only ingredient. Wilson, W.K., et al.Preparation …, pp. 7–8.
 Section Index Section Index |