TECHNICAL EXAMINATION OF THE CLASSICAL BRONZE HORSE FROM THE METROPOLITAN MUSEUM OF ARTKate C. Lefferts, Lawrence J. Majewski, Edward V. Sayre, Pieter Meyers, R.M. Organ , C.S. Smith , Edward V. Sayre , Robert H. Brill , I. Lynus Barnes , Thomas J. Murphy , & Frederick R. Matson
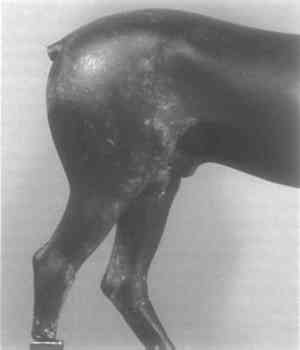
2 SURFACE ACCRETIONS AND CORROSION PRODUCTSFairly heavy deposits of whitish accretions lie over much of the surface. They form an almost continuous outer layer on the mane and are heavy over the top of the right foreleg, around the plugs, inside the mouth and below the mouth and chin. The deposit on the mane was determined to contain sand and calcium carbonate by microchemical testing. Underneath it there are deposits of green over red corrosion products. Many investigations have shown that the natural corrosion products on archaeological bronzes include a red copper oxide (cuprite) layer under a green layer of a copper hydroxy carbonate (malachite) or a copper hydroxy chloride (atacamite or paratacamite). X-ray diffraction measurements confirm the presence of cuprite and of copper hydroxy chloride. A thick deposit on the tongue was determined microchemically to be calcium hydroxy carbonate. Below it also lie green and red corrosion products. X-ray diffraction analysis carried out at the Conservation Center of the Institute of Fine Arts, New York University, identified the deposit on the right foreleg to be calcite (calcium carbonate) and a combination of calcite and silicon dioxide The bronze is coated extensively with a smooth, thin, well-attached layer of brownish black corrosion product which appears in part to have been removed mechanically, in some areas down to the surface of the metal, notably on the left foreleg. A sample taken from the right rear leg, similar in appearance to the crust on the nose, was determined by x-ray diffraction in the Analytical-Conservation Laboratory of the Smithsonian Institution to be bornite (Cu5FeS4), a natural mineral difficult to manufacture artificially. Its presence is not usual on ancient bronze. X-ray diffraction carried out at the Conservation Center showed the black deposit on the left rear leg also to contain bornite together with copper and cuprite, and that from under the right rear leg to be djurleite (Cu1.96S), a natural mineral. The finding of these minerals is consistent with a long-term natural corrosion process. X-ray diffraction analysis of a green sample from the right flank showed it to be paratacamite. A later x-ray diffraction analysis of a green sample from the right flank identified it as a mixture of paratacaamite and atacamite. On the inner wall of the bronze where it was drilled at the square plug on the leg, on the edge of a bronze flow at a depth of 4 to 5 mm, there is a green corrosion product which was analyzed by x-ray diffraction to be a mixture of paratacamite and atacamite. The analyst, Seymour Z. Lewin, claims that if the patina contains atacamite or a mixture of atacamite and paratacamite, then the probability is very great that the patina is false.8 However, examinations of many bronzes of unquestioned antiquity at the British Museum9 and at the Metropolitan Museum10 have shown that genuine, naturally formed corrosion often contains a mixture of paratacamite and atacamite. Furthermore, experiments by Pieter Meyers and E.V. Sayre have demonstrated that when copper metal is immersed for a period of days in aerated sodium chloride solutions buffered to an acidity similar
There is no evidence that natural corrosion products have been mechanically applied to the surface. Professional foundrymen John Spring and Bruno Bearzi agreed that the corrosion products were not artificially generated. Bearzi considered that the corrosion products as observed on the horse could not have formed in less than 200 years. The black patina is very hard, and he thought it could not be formed in such a hard layer artificially.3 R.M. Organ, Chief of the Conservation-Analytical Laboratory, Smithsonian Institution, studied the horse. For his metallurgical examination two specimens were taken, a core drilling from the bottom of the right rear leg and a cross section obtained by enlarging a hole already hand-drilled into the barrel of the horse (see Appendix I). The specimen from the leg revealed a dendritic structure containing interdendritic cavities and globules of lead. Although no major corrosion crust remained on this specimen, the presence of pits indicated that mineralization penetrated into the metal structure to a depth of 0.3 mm. At high magnification some replacement of a metallic phase by mineral was observed which left no doubt that some corrosion processes have taken place within the metal itself and that the mineral has not merely formed a surface layer. The cross section from the barrel, about 10 mm long, revealed a negligibly thin corrosion crust on the outer surface. The inner surface was not mineralized to any greater degree, although it did possess a number of pits that should have contained mineral if corrosion had occurred. Hairline cracks which normally accompany mineralization were absent from both samples. Interdentritic cavities free of corrosion products and very small separated globules of lead indicated that the metal had chilled unexpectedly rapidly. Organ concluded “that the results of this particular examination do little to favor a belief in the antiquity of the samples. Nevertheless, on occasion, objects that carry very little mineralization have been regarded by archaeologists as genuine and many thousands of years old.” From inside the hole under the square plug in the left rear leg, loose reddish black corrosion products and several small black pieces from the leg armature which were attracted to a magnet were shaken out. These on close inspection turned out to be a conglomerate of magnetic iron oxide (Fe2O4) mixed with core. The plug was examined metallurgically by C.S. Smith (see Appendix II). He reported that it shows a roughness on some edges which suggests ancient filing and that the dendritic structure, although fine, is characteristic for any normal cast bronze. He noted the presence of both intergranular slip-band corrosion and surface corrosion on grain boundaries near the bronze surface. He commented upon the relatively small amount of corrosion on the inner surface but suggested that possibly metal contact between the bronze and the iron armature and chaplets within the core might have protected the bronze until the iron was completely corroded. Such electrogalvanic protection could indeed have delayed overall corrosion of the bronze. Although the metal of the bronze horse is not deeply penetrated by the corrosion, other ancient bronzes of unquestioned authenticity have a similar degree of surface alteration. After examining the horse visually, Nicolas Yalouris, Inspector General of Antiquities, Minister of Culture and Science, Athens, and Director of the National Archaeological Museum in Athens, stated that many of the bronzes from Olympia showed no more degree of corrosion. Certainly the nature, ordering and |