Treatment of Two Nineteenth-Century Chromolithographs: an Approach to Reduction of Magnesium Bicarbonate Deposits
by Elissa M. O'LoughlinIntroduction
Two chromolithographs with a disfiguring white surface deposit were received for conservation treatment. They were two impressions of the same chromolithograph depicting a young girl seated on a classical column holding a box of Hires Root Beer powder mix, and signed on the stone at lower center "Gast N.Y. & St. L. Copyright 1889"1. Reducing the white deposits on these prints was a challenge requiring identification of the unknown deposit and design of an appropriate treatment.
Examination
The chromolithographs exhibited structure characteristic of the period. the heavyweight off-white paper was prepared with white pigmented coating. a surface glaze over the media, probably a gum, was suspected but could not be seen due to the deposit. the deposit, which both chromolithographs exhibited to the same degree, appeared whitish, dull, hazy, and looked much heavier over areas of dark media. Upon microscopic examination however, the deposit was seen to cover areas of lighter media as well. Removal or reduction of the deposit seemed indicated, but the coated paper structure, heavy mechanical damage, and presence of potentially soluble media complicated the design of a treatment approach. Both chromolithographs had been folded at some time in the past, which caused breaks in the coated surface of the paper and losses in the image areas. Inscriptions in blue grease pencil on the verve, and violet stamp pad ink prints were present on the recto of both prints.
Testing
pH
The pH of the surface of one of the chromolithographs, tested in an area of heavy deposit with a Merck pH indicator strip, indicated pH 9.5-10.0. the elevated pH suggested that the deposit was an alkaline salt, possibly magnesium or calcium. While there were no treatment records available for these chromolithographs, it was suspected the deposits could be the result of a well intended but uninformed attempt to deacidify them. Further testing was performed based on this hypothesis.
Figure 1. Both chromolithographs before treatment. Lithograph "B" (left) is in much worse condition than lithograph "A" (right),and was treated first.
X-RAY Fluorescence Spectroscopy
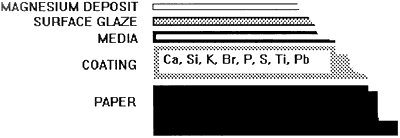
A small detached portion of one corner of chromolithograph "B" was analyzed by X--ray fluorescence. a cross section shown in figure 2 illustrates the structure of the chromolithographs and components of the coating as detected by the XRF. Curiously, no magnesium was detected by the x-ray; this result does not discount the presence of small amounts of magnesium, but suggests that a larger quantity of magnesium is necessary to be measured by the x-ray. Also, the atomic number of magnesium poses difficulty in detection by XRF. Subsequently, magnesium was easily detected in a filter paper sample saturated with a 0.06 molar solution of magnesium bicarbonate (standard aqueous deacidification solution).
Figure 2. the structure of the chromolithographs. the elements in the coating are listed in order bases on the quality present as detected by XRF.
Deposit Solubility
The deposit was tested for solubility in deionized water and in a solution of carbonic acid in deionized water. the deionized water was drawn directly from the deionizing column and measured ph 5.0-5.5. a the carbonic acid solution was made by bubbling carbon dioxide through a diffusing stone into deionized water. This solution also measured pH 5.0-5.5. a poultice method for testing was used in preference to direct application of water. the coated paper and its condition ruled out the possibility of immersion.
A small piece of filter paper (Whatman chromatography #1) was dampened with deionized water. the paper was then placed on the chromolithograph in an area of heavy deposit. the Filter paper was weighted lightly and left for approximately 5 minutes. the carbonic acid solution was Tested in the same manner. the results were evaluated after the test area was allowed to dry. a reduction in the deposit based on visual observation was used to determine effectiveness. Increasingly longer times of exposure to both test liquids resulted in further reduction of the deposit, however, the deionized water appeared to work much more quickly. the aggressive nature of deionized water in dissolving ions is well documented2 and it seems probable that this aggressiveness was responsible for deposit removal in this context.
Testing of Media and of Soiled Areas
All media recto and verve were tested for solubility on prolonged contact with deionized water applied via dampened filter paper. All appeared stable and unchanged after successively longer applications of the filter paper. It is interesting to note that the high pH created by the surface deposit did not appear to affect the solubility of the media. Areas where engrained dirt was not removable were also tested for solubility over prolonged period using the same method. Surface soil was tested for ease of removal with a soft brush and vinyl eraser crumbs.
Treatment Design
The goal of treatment was to reduce the surface deposit in order to improve the visual appearance of the chromolithographs, and to lower the pH to approximately neutral. the general approach for removal of the deposit was based on a treatment developed by Craigen Bowen of the Fogg Museum for reduction of sodium chloride crystals in a woodcut by E.L. Kirchner3. One slight variation to her technique was made by using filter paper instead of blotting paper as the vehicle for the deionized water. Because the deposit had not yet been definitively identified, the bulk of it which would dissolve into the filter paper could be differentiated from the known components of the filter paper, and subsequently analyzed. One of the chromolithographs ("B") was in much worse condition than the other ("AN) and had detached pieces and multiple corner folds. the difference in condition between the two influenced the order in which treatment was performed. It was decided that chromolithograph "B" would undergo treatment first; treatment of the second chromolithograph "A" would proceed based on post treatment evaluation of "B".
Treatment Procedure
Chromolithograph "B" was placed recto up on a sheet of 3 mil polyester film. a piece of spun-bonded polyester was placed on top of it to act as a release layer, and a piece of filter paper dampened by spray application of deionized water was placed on top. a lightweight acrylic sheet was placed over the sandwich, and a timer was set for 1 hour. After the first hour, new polyester web and new filter paper dampened to the same degree, were substituted. This procedure was repeated five times for a total of 6 hours. As sheets of filter paper were removed, they were tested for pH in several locations using a surface pH electrode. the testing showed small upward rises of 0.10-0.50 in the pH of the filter paper. at the end of 6 hours, the surface (recto) of the chromolithograph, tested with a contact electrode, measured pH 7.0. Drying was performed overnight between blotters under light weight. the apparent reduction of the deposit as indicated by the lowered pH was confirmed by visual examination after drying. the visual effect of the deposit was completely eliminated, making it possible to look for the suspected surface glaze. a test was performed to determine if a glaze would reform, and thus become more glossy. an ethanol chamber was inverted over a small area of the surface of the chromolithograph for approximately 30 minutes. Visual examination showed increased gloss in the test area, so a chamber was created to expose the chromolithograph to ethanol vapors. After about 1 hour in the chamber, the results were visible. Areas of darker media had attained depth, and an overall sheen could be seen when the chromolithograph was viewed at a low angle. the treatment was completed by mending tears and filling losses with Japanese paper and wheat starch paste. No inpainting or color compensation was done by agreement with the archivist.
Figure 3. Spray application of the filter paper just prior to application to the chromolithograph recto.
Figure 4. (TOP) Chromolithograph "B" on the left after deposit reduction treatment and chromolithograph "A" on the right before treatment.
Figure 5. (BOTTOM) Both chromolithographs after treatment.
Testing for Magnesium
After the treatment was completed, the filter paper sheets were subjected to testing. a spot test and a microchemical test were both investigated for use as nondestructive methods for identifying magnesium. Atomic absorption was also used.
Quinalizarin
The spot test consists of a staining a sample with a solution of quinalizarin (1,2,5,8 tetrahydroxyanthraquinone) in ethanol. a blue lake is formed on magnesium hydroxide by quinalizarin, the intensity of which is proportional to concentration. a violet colored lake forms on magnesium carbonate. Standard aqueous deacidification solution (0.06 molar solution of magnesium bicarbonate in deionized water) turns dark violet-blue by addition of quinalizarin. a proportionately weaker solution turns a blue that is several degrees lighter. a 2% solution of quinalizarin in ethanol was prepared4 and used to stain the sheets of filter paper which were retained from the treatment. the filter paper quickly changed to an overall violet color with areas of darker intensity corresponding to the tears and folds in the chromolithograph. This indicates greater absorption of the magnesium in the paper fibers which were exposed by the breaks in the coated surface. a sheet of filter paper which had been immersed in standard aqueous deacidification solution (which became violet), and a piece of filter paper (which stained the same color as the orange quinalizarin solution) were used as controls.
Figure 6. the lake formed by quinalizarin on magnesium hydroxide is dark blue. the magnesium carbonate lake is violet, and lighter in intensity.
Sodium Phosphate
The microchemical test for magnesium by means of sodium phosphate in an ammoniacal solution is described in Chamot and Mason5". the sample, in this case, fibers from the retained filter paper, were placed on a slide in a drop of deionized water. a few tiny fragments of NH4CI were added and the droplet was stirred with a glass rod. Several fragments of citric acid were added, then the slide was gently warmed. a fragment of Na2PO4 was added and the slide warmed again. After addition of a drop of NH4OH, crystallization began. Viewed at 100x, long dendritic shapes began to form. Rectangular crystals, resembling envelopes, resulted as the product, NH4MgPO4·H2O formed. This test was used on a variety of papers as a determinant for magnesium. It was found to be so extremely sensitive that most papers tested contained enough magnesium to produce crystals. These results suggest that this test method is too sensitive to indicate the presence of magnesium in quantities associated with previous deacidification.
Figure 7. Crystals of NH4MgPO4· H2O associated with paper fibers viewed at 200x. the crystals resemble envelopes or tiny rooftops.
Atomic Absorption
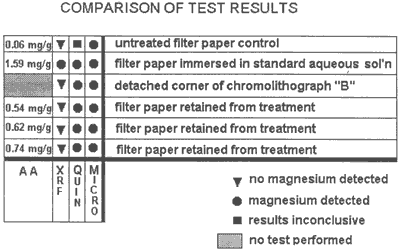
Atomic absorption was used to evaluate the results of the other test methods. Five samples were tested. the results are expressed in milligrams of magnesium per gram of paper (mg/g).
| (mg/g) | |
| filter paper immersed in non-aqueous deacidification solution | 1.59 |
| filter paper retained from treatment | 0.54 |
| filter paper retained from treatment | 0.62 |
| filter paper retained from treatment | 0.74 |
| untreated filter paper control | 0.06 |
It is interesting to note that the sodium phosphate test was able to detect magnesium in such small amounts in the filter paper control. the quinalizarin did not function well at such low concentrations possibly because its bright orange color masks lighter tones of violet. Also, the XRF did not identify magnesium in the control.
Conclusions
The chance to undertake this unusual treatment allowed for the investigation of methods for reduction of the surface deposit and for the evaluation of four methods to detect presence of a magnesium compound by non-destructive means.
COMPARISON OF TEST RESULTS
Figure 8. The chart shows comparative uses of each of the four test methods.
Acknowledgements
I would like to thank my colleagues in the Document Conservation Branch of the National Archives and Records Administration, especially Susan Lee-Bechtold, William K. Wilson, and Kitty Nicholson. Skip Palenik of McCrone Associates advised on microchemical and spot test methods. Erica Mosier, Dianne van der Reyden, and Karen Tidwell all generously contributed their help and guidance.
Notes
1. RG 21, Records of the District Courts of the United States, Hire Case # 3968 Filed Dec. 24, 1896 (6NN).
2. Tang, Lucia and Norvell M.M. Jones "refThe Effects of Wash Water Quality on the Aging Characteristics of Paper,"ref JAIC 18 (1979) pp 61-81. Wash. D.C.
3. Weston (Bower), Craigen, "refTreatment of a Salt Impregnated Woodcut"ref JAIC 18 (1979) pp 95-107.Wash. D.C.
4. The quinalizarin solution is prepared by addition of quinalizarin powder to ethanol, and then filtering it through a fine sieve or filter paper.
5. Handbook of Chemical Microscopy. Chamot, E.M. and Clyde W. Mason. John Wiley and Sons, N.Y. 1931 Vol. 2 pp. 121-123.
6. The pH of the manuscript varied from pH 5.0 to pH 7.0. the surface felt powdery, and previous exposure to water was evident in feathering of the iron gall ink in areas of heavy application.
Elissa M. O'LoughlinPreservation Policy and Services Division, Document Conservation Branch
National Archives and Records Administration
Publication History
Received: Fall 1992
Paper delivered at the Book and Paper specialty group session, AIC 20th Annual Meeting, June 2-7, 1992, Buffalo, NY.
Papers for the specialty group session are selected by committee, based on abstracts and there has been no further peer review. Papers are received by the compiler in the Fall following the meeting and the author is welcome to make revisions, minor or major.