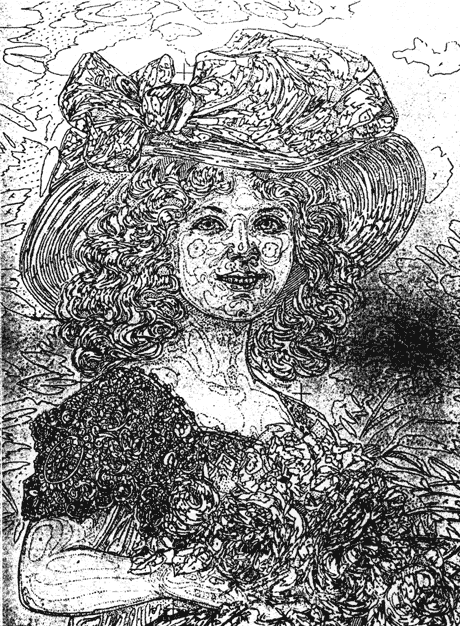The Technology and Treatment of an Embossed, Chromolithographic "Mechanical" Victorian Valentine Card
by Erika Mosier, Dianne van der Reyden, and Mary BakerI. Introduction
The Smithsonian Institution houses the National Valentine Collection. One Valentine, a stand-up "mechanical" card, required removal of adhesive on its base and verve (Figs. 1, 11, and 12). the card is composed of embossed, die-cut chromolithograph prints or "scraps" on glazed, coated papers. in combining these various characteristics, which will be discussed further in this article, this Valentine is in essence the product of two parallel innovations in the mid-19th century: the increase in mass communication through the exchange of greeting cards, and the development of mass color printing, through chromolithography, called the democratic art by Marzio (30). Unfortunately, the literature on greeting cards (1, 2, 5, 6, 11, 13, 14, 19, 20, 21, 23, 25, 27, 33, 38, 39, 41), and chromolithography (4, 7, 8, 9, 15, 16, 17, 22, 24, 28, 29, 30, 32, 33, 34, 35, 36, 37, 43, 44, 45, 46) provides little information of use to conservators on the manufacture, composition, or deterioration of chromolithographic cards. Virtually no literature exists on the analysis or conservation treatment of chromolithographs, although there are some relevant references on the conservation of pigment-coated papers (3, 12, 18, 47, 48). the following brief history of Valentine cards, and the technology used to produce them, represents a review of the available Literature.
Fig. la. Victorian Mechanical Valentine Recto, Before Treatment (Property of the Archives Center, National Museum of American History, Smithsonian Institution)
Fig. 1b Xeroradiograph of Valentine, Showing Hinging Tabs and Damage from the Adhesive including Cracking and Losses, Before Treatment
Fig. 2. Preparatory Drawing for Embossed Lithograph (From: Pieske, Christa. Das ABC des Luxuspapiers: Herstellung, Verarbeitung und Gebrauch 1860 bis 1930. Museum fur Deutsche Volkskunde Berlin, Staatliche Museen Preussischer Kulturbesitz, Berlin, 1983, p. 35)
A Brief History of Valentine Cards
The observance of Valentine's Day is over seventeen centuries old, originating after "the Christian Valentine was beaten by clubs and beheaded, at the time of the great heathen festival of love and purification" (41). a legend asserts that Valentine befriended his jailor's daughter. and on the eve of his execution sent her a farewell note signed "From your Valentine" (5). From the 15th to the 18th century, the simple text of early Valentines became increasingly embellished by hand-colored drawings with motifs of love knots, hearts, and flowers. in the early 19th century, hand-made Valentines featured hand-colored engravings and cut-out work, such as "cob-web" designs that could be lifted by a thread to expose a picture, verse, or a lock of hair (41). Embossing could be done by hammering cards with metal dies (Fig. 4). In 1834, Joseph Addenbrooke of the English firm of Dobbs came up with the idea of embossing a sheet of paper and then filing off the raised part of the paper to create a lace-like effect (38, 41). By the mid-19th century, the exchange and production of greeting cards increased. owing to, the reformation of the English postal system and refinements in print-making technology in Germany and England. the establishment of the Uniform Penny Post allowed inexpensive and efficient exchange of cards, while the mass production of inexpensive "penny plain. two-pence colored" prints enabled people to buy and send more cards (6, 38). Popular innovations included "mechanical" or movable cards. embossed and lace-paper cards and die-cut cards (21, 27). Movable cards might contained doors or shutters that opened, or movable parts which might be riveted or attached by pull tabs, as in the case of one humorous 1850s Valentine that exposes a lady's pantaloons when her hoop-shirt is pulled by tabs. Other attachments included complicated hinges arranged to support stand-up cards (Fig. 1b).
After the middle of the 19th century embossing and die-cutting became more automated. Reportedly. Ester Howland an American who employed women to assemble Valentines from embossed and perforated lace-paper blanks colored pictures. and other trimmings desired to do away with time-consuming hand-cutting and contacted a German Firm to create embossing and cutting-dies (27.39). This type of process involves executing a design (Fig. 2) on a brass die and making a counter-die (Fig. 3) of a resilient material such as gutta percha. special embossing compounds plaster of Paris. tissue paper or kraft paper and fish glue. a cutting-die is made by encircling the contours of mounted, trimmed proof with a steel rule which is then mounted onto a metal plate. having cork or sponge rubber blocks protruding to eject the die-cut sheet (17). Designs could be embossed then die-cut or embossed and cut at the same thee. This procedure was used to produce "scraps" or die-cut printed images joined together by tabs or "ladders" (Fig. 5) into a sheet that could be cut and assembled by the consumer (2, 14, 39).
A Brief History of Chromolithography: Materials and Degradation
The popularity of greeting cards was aided by developments in the middle of the 19th century in chromolithography. Prior to this time lithographic prints were made by inking a drawing on a porous limestone block covering it with paper, and running the ensemble through a press (4, 16). Lithographic prints could then be hand-colored or tinted. There are many ways to define the term chromolithography. but the definition most generally accepted refers to a lithograph printed in at least three colors each applied from a separate stone (30). Alois Senefelder is credited as the first printer to do this although he did not use the term chromolithography. the French version of the term "chromolithographie" first appears in a 1837 French patent by Godefry Engelmann. while "chrome-lithography" was coined in England two years later by Charles Hullmandel (30). Louis Prang is credited as the first to apply the designation "chromos." according to an 1875 London journal (27, 30). the Germans, who were responsible for the most elaborate developments in embossing and varnishing of chromolithographs. called them "Glanzbiler" (gloss pictures) and "Oleographs" (2, 30, 38, 39).
Four periods of development have been distinguished. beginning with low embossed reliefs produced by Germany in the first halt of the 1800's (2). Between 1850 and 1880. creeper embossing was produced by a "rise and fall" method of dropping a die-punch from a height onto a sheet. Following the introduction of steam presses in 1860. steel plates began to replace lithographic stone t´-,r commercial printing especially between 1880 and 1900. From 1900 to 1920 techniques using color separation cameras and photographic plates dominated the commercial printing of " chromolithographs. " Innovations in the mass multiple-color printing of chromolithographs included not only developments in the technologies of printing embossing and die-cutting. but also developments in the materials of the paper. inks and glazing varnishes.
Chromolithographs could be printed on coated and/or calendered paper. Prang. for the reproduction of fine arts paintings, embossed a canvas texture onto highly calendered paper (25). "Enamelled paper" could be made with a coating of flake (lead) or Kremnitz white. light-colored glue. and a little alum popular with European firms like Raphael Tuck and Ernest Nister (25).2 in the last quarter of the 19th century, the development of the halftone photomechanical process by George Meisenback initiated a revolution in the coating of papers (29). Prior to the 19th century. coatings were applied by hand-brushing. in the mid- 19th century (1852) the process was automated so that the coatings could be applied in a correct weight to a continuous web of paper and automatically brushed to a level surface, as illustrated in Fig. 6. Coating was applied by a splatter brush at starting point A. grossly smoothed by a coarse brush at B, refined by a firm brush at C. and finished by a light brushing with badger hairbrushes at end point D. to dry the coating, the paper was festooned on wooden sticks and slowly carried through a long drying chamber maintained at low temperatures. This type of coater produced paper of surprisingly good quality, but of poor uniformity. in the late 19th century (1880). paper could be coated on both sides at the same time (Fig. 7). in the first quarter of the 20th century (1920). brushes were replaced by roll-spreading bars rotating at high speeds and lightly touching the paper. Roll-spreading coaters are still in operation. A later method used an air-knife (Fig. 8). which produced a stream of air from a transverse nozzle to control the coating weight and spread the coating in one simple operation, leaving a smooth surface (Fig. 9). the next instrument developed was the blade-coater. named for a flexible blade running against the paper, which is backed by a rubber roll (Fig. 10) (29).
Fig 3a & b. Embossing and Steel Rule Cutting Dies (From: Hackleman, Charles W., Commercial Engraving and Printing. Indianapolis: Commercial Engraving Publishing Company, 1921)
Fig. 4. Tools for embossing paper flowers motifs, c. 1900 from Josef Heller, Walldurn. Property of the Heimatmuseum, Walldrun. (From: Pieske, Christa. Das ABC des Luxuspapiers: Herstellung Verarbeitung und Gebrauch 1860 bis 1930. Museum fur Deutsche Volkskunde Berlin, Staatliche Museen Preussischer Kulturbesitz, Berlin, 1983. P. 197).
Fig. 5. Detail from SI Victorian Valentine, showing angle wing with "ladder" tab attached.
In the latter part of the 19th century. chromolithographs were printed with pigments ground boiled linseed oil, which printers called varnish (30). a printed chromolithograph, before embossing in the press, could be glazed with colloidal glue, gelatin. gum or alcohol or turpentine soluble "varnish." the dried glaze enabled the paper to stretch during embossing without cracking the printing ink, while saturating the colors and enhancing gloss (1, 23, 37).
Typical forms of deterioration found by the authors in chromolithographs include yellowing. cracking, and flaking of the glaze. sometimes taking the printing ink with it. This flaking can also include the coating layer, especially when blocking occurs in books. such as scrapbook albums. which were a popular way to display "scraps," particularly at the turn of the century (2, 6, 14). These scrapbooks also often show staining on pages on which "scraps" are mounted from the adhesive used. and staining on adjacent pages, possibly from acidic surface glazes of the "scraps.'
Unfortunately, the advent of world War in 1914 resulted in an interruption in the production of chromolithographic cards by German firms. By the end of World War 11, many of the records on the technology of chromolithographic cards, both in Germany and England, had been destroyed (2, 6, 23, 39)
II Removal of Adhesive From a Victorian Valentine
The Victorian stand-up Valentine (Fig. 1) came into the Conservation Analytical Lab (CAL) for removal of a thick accumulation of adhesive, which covered the verso (Fig. 11). Closer examination revealed adhesive also on the recto base of the card (Fig. 12), suggesting that the card may once have been mounted in an opened position. the recto and verve both had massive areas of skinning which weakened the paper support. In addition, the adhesive was extremely yellowed, brittle, and cupped in many areas. causing continued discoloration and loss of surface on troth the front and hack of the card. Consequently, removal of the adhesive was necessary not only for the appearance. but also for the stabilization of the card. However, initial attempts to identify the verso adhesive. which varied in thickness, color. and consistency, proved inconclusive. Eventually, FTIR analysis revealed that the verso was covered with two adhesives: an initial layer of hide glue was obscured by a subsequent layer of rubber cement. The presence of hide glue on the recto base of the card suggested that at one time the base and verso of the card had been attached by hide glue to some mounting system. After the card became detached for some reason,. rubber cement was applied over the verso for reattachment to a now vanished mount.
Fig. 6. Single-Surface Brush-Coating Process (From: Marchessault, R. H. and Christen Skaar. Surfaces and Coatings Related to Paper and Wood. Syracuse: Syracuse University Press, 1967, p. 314)
Fig. 7. Double-Surface Brush-Coating Process (From Marchessault, etc., p. 314)
Fig. 8 & 9. Air-Blade or Air-Knife Coating Process and Detail of Brush-like Air Stream (From Marchessault, etc., p. 316)
Fig. 10. Blade-Coating Process (From Marchessault, etc., p. 319)
Fig. 11a. Verso of SI Valentine, Showing Multiple Layers of Adhesives (Rubber Cement on Top of Hide Glue), Discoloration, Cracking, and Delamination Before Treatment
Fig. 11b. Detail. Partially Cleaned
Fig. 11c. Verso of SI Valentine After Treatment to Remove Multiple Adhesive Layers
Fig. 12a. Base of SI Valentine, Before Treatment
Fig. 12b. Detail of Base Showing Hide Glue Adhesive, Discoloration, Cracking, Delamination, and Surface Loss, Before Treatment
Fig. 12c. Detail of Base After Treatment to Remove Adhesive and Consolidate Delaminated Surfaces.
Once the adhesives had been identified. a treatment protocol could be designed. The basic treatment, summarized here briefly for the adhesive removal only, required first that the top layer of the rubber cement on the verso be removed by swabbing with toluene (Fig. 11b). Following this the subsequently exposed hide glue was softened with an agarose "pack"3 and removed mechanically (Fig. 11c). On the recto the removal of hide glue proved more problematic since the chromolithograph was printed on coated paper and the contracting glue had cupped areas of the coating (Fig. 12b). Poulticing was not effectively controllable in these areas and after testing many alternatives the safest procedure required the use of a Protease (Pronase) enzyme solution.4 This solution worked quickly to break up the adhesive, thereby limiting moisture exposure to the coated and glazed surface of the chromolithograph5 and reducing the possibility of dissolving the coating. The solution was applied under a microscope with a 000-fine brush which could then be used to remove the hulk of the swollen and softened adhesive mechanically. During this process the cupped coating relaxed and could be eased back into plane with the brush. using residual adhesive as a consolidant (Fig. 12c).
All areas treated with enzyme were swabbed lightly with ethanol to increase denaturation as a caution against reactivation. These same areas. as well as those treated with agarose. were then swabbed with deionized water in a effort to remove residual enzymes or agarose.
Treatments of this nature, while very successful, none-the-less have stimulated questions about whether techniques using solvents, applied in various ways to pigment-coated or chromolithographic surfaces, could cause 1) cracking or loss of the coating on a microscopic scale; 2) softening of the coating: and 3) change in general appearance (i.e. gloss or color). In response to these questions, several research projects have been initiated. and some of the preliminary findings have been prepared for publication (47). The types of projects and some general results are summarized below.
III Research
The following 3 studies are part of on-going research to a) characterize types of pigment-coated papers and b) compare the effects of solvents applied to such papers by different application techniques. The three studies reported here each focus on the effect of various applications of solvents on surface appearance of three separate classes of pigment-coated papers. The three classes of pigment-coated papers currently under study are 1) traditionally hand-coated papers 2) glazed chromolithographs and 3) modern machine-coated papers (App. A). The treatment procedures compared in the current report are the application of water as a solution or as a poultice, followed by air drying or blotter drying (App. B and C). the effects on surface appearance were evaluated by SEM imaging, SEM/EDS, gloss and color (App. D).
Study 1: Effects of solvents and application techniques on traditionally hand-coated papers: A survey of traditional recipes provided the basic information for sample preparation (49).
The sample papers were hand-coated with combinations of calcium carbonate. zinc oxide and barium sulfate in binders of gum, glue, and acrylic resin (App. A.1). A machine-coated paper composed of barium sulfate and calcium carbonate in an acrylic binder was examined and tested as a comparison for a modern pigment-coated paper. The treatment procedures were selected to test the effects of 4 solvents—water, ethanol, acetone, and toluene--applied in 3 ways—immersion, poultice (diatomaceous earth). and suction disk—to each of the papers (App. B.1). The results indicated that, among other things, the aqueous poultice applications used in this study could cause cracking of some pigment-coated surfaces, especially the machine-coated sample (3, 47).
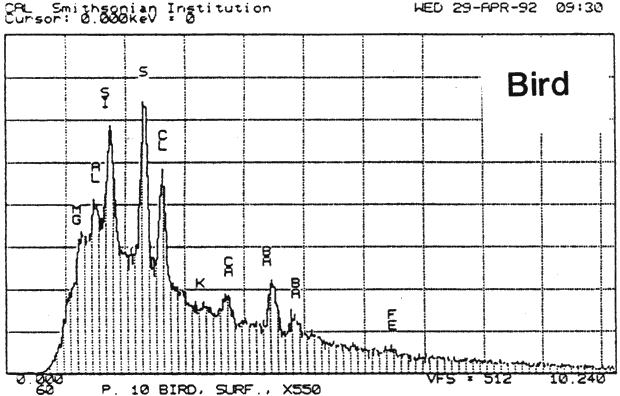
Study 2: Effects of water on glazed chromolithographs: SEM/EDS analysis of a selection of historically authentic, glazed chromolithographs from a scrapbook donated to CAL indicated that the chromolithographs were made of primarily clay and blanc fixe, although they could be divided into three groups based on elemental compositions (Fig. 13a, App. A.2). Chromolithographs in Group I consist primarily of clay (Al, Si) and barium sulfate (Ba, S). Group II consist primarily of clay (Al, Si, K) and barium sulfate (Ba, S). Group III consists primarily of clay (Al, Si, K) and barium sulfate (Ba, S) and gypsum or talc (Ca). The binder, identified by FTIR, consisted primarily of casein. Treatment testing focused on the effect of water brushed onto the surface, or glaze, of selected authentic chromolithographs from the scrapbook (App. B.2). The results of this study indicated that some aqueous applications could cause opening of cracks and additional cracking of glazes (Figs. 17a & b). However, treatment trials on the glazed chromolithographs from the scrapbook indicated that pre-existing mechanical damage could interfere with evaluation of changes. to avoid the variability of older, glazed chromolithographs, study 3 investigated modern unprinted, unglazed machine-coated paper. recommended by manufacturers for chromolithographs and silverpoint drawings.
Study 3: Effects of water on modern machine-coated papers: SEM/EDS analysis of selected modern machine-coated papers indicated that they could be divided into three groups based on elemental analysis (Fig. 13b, App. A.3). Group I is composed primarily of barium sulfate (Ba, S) and talc (Si, Ca). Group II is composed primarily of clay (Al, Si, K) and gypsum (Ca, S). Group Ill is composed primarily of clay (Al. Si) and talc (Ti, Ca) or titanium dioxide and calcium carbonate. To select control papers for testing, the compositions of these papers were compared with the pigment coatings (powdered) from the authentic, scrapbook chromolithographs (Figs. 13a & b, App. A, Table 2 & 3). Based on these comparisons, as well as FTIR analysis, three modern machine-coated papers were selected for treatment trials and testing, and are described below under the designations "S," "L," and "B.'
| PAPER "S" | PAPER "L" | PAPER "B" | ||
| FIBER | 40% chemical, 50% mechanical wood pulp 10% rag (est.) | 85% chemical softwood, with some chem. hardwood 15% rag (est.) | 100% ground hard wood and softwood(estimate) | |
| INORGANIC PIGMENTS- | EDS | Ti, Si, Al, Ca | Si, Al, Ca. S. K | Ba, S, Ca |
| FTIR | clay, calcium carbonate | clay | sulfate, some clay | |
| ORGANIC BINDER | styrene acrylic resin | protein, acrylic | protein, resin | |
| SURFACE FINISH | matte, slightly rough | very glossy, smooth | matte, smooth | |
Fig. 13a (Victorian Valentine): SEM/EDS Analysis of Glazed Chromolithographic Cards
Fig. 13a (Friendship Card): SEM/EDS Analysis of Glazed Chromolithographic Cards
Fig. 13a (Red Flower powder): SEM/EDS Analysis of Glazed Chromolithographic Cards
Fig. 13a (Large Bunch powder): SEM/EDS Analysis of Glazed Chromolithographic Cards
Fig. 13a (Ever Thine powder): SEM/EDS Analysis of Glazed Chromolithographic Cards
Fig. 13a (Bird): SEM/EDS Analysis of Glazed Chromolithographic Cards
(Note that SEM/EDS of chromolithographs differs depending on whether sample is prepares] by powdering or analyzed directly on surface or cross-sections, which may be contaminated by some of the surface pigments and glazing)
Fig. 13b (B): SEM/EDS Analysis of Several Modern Pigment-Coated Papers Manufactured for Chromolithographic Printing and Metal Point Drawing.
Fig. 13b (L) : SEM/EDS Analysis of Several Modern Pigment-Coated Papers Manufactured for Chromolithographic Printing and Metal Point Drawing.
Fig. 13b (K) : SEM/EDS Analysis of Several Modern Pigment-Coated Papers ManufaKtured for Chromolithographic Printing and Metal Point Drawing.
Fig. 13b (S) : SEM/EDS Analysis of Several Modern Pigment-Coated Papers Manufactured for Chromolithographic Printing and Metal Point Drawing.
Fig. 13b (F) : SEM/EDS Analysis of Several Modern Pigment-Coated Papers Manufactured for Chromolithographic Printing and Metal Point Drawing.
Fig. 13b (C) : SEM/EDS Analysis of Several Modern Pigment-Coated Papers Manufactured for Chromolithographic Printing and Metal Point Drawing.
Fig. 13b (R) : SEM/EDS Analysis of Several Modern Pigment-Coated Papers Manufactured for Chromolithographic Printing and Metal Point Drawing.
Fig. 13b (Q) : SEM/EDS Analysis of Several Modern Pigment-Coated Papers Manufactured for Chromolithographic Printing and Metal Point Drawing.
FTIR Analysis of Binder and Pigment of Two "Historic" Chromolithographic Cards
FTIR Analysis of Binder in Three Modern Pigment-Coated Papers Manufactured for Chromolithographic Printing and Metal Point Drawing
| Fig. 15: Colorimetry (DELTA) | Fig. 16: Gloss (DELTA) |
|---|---|

| 
|

| 
|

| 
|
The treatment trials compared effects of water applied by immersion as opposed to poulticing with a damp blotter. as might be used to remove accretions. These two treatment techniques were selected to represent differences in amount of solvent absorption. exposure time to solvent, and direction of solvent application and evaporation. for example, Immersion might result in greater penetration, longer exposure. and lateral movements of compounds as compared to poulticing. Changes in the papers after treatment were assessed by measuring appearance properties, such as color and gloss (Figs. 15 & 16). and observing any changes to fibers. binders' pigments and surface affects.
There were two variations of the treatments comparing the effects of immersion to blotter poulticing (App. B.3). the only difference between them was that Variation (a) used smaller samples (c. one centimeter square) and a spun-woven fibrous polyester web support during treatment. while Variation (b) used larger samples (c. 8" X 10") and a non-woven smooth polyester wet, support. for both variations~ half of the samples were exposed to a five minute immersion treatment in deionized water. while the other half of the samples were poulticed with a damp blotter (unbuffered) for five minutes. Half of the treated samples were air dried and the other half were dried in a blotter (unbuffered ) press (App. C).
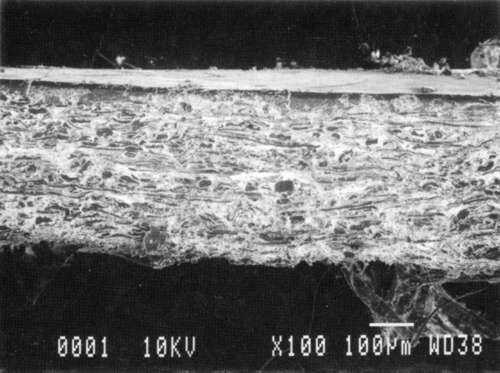
Results from Variation (a) indicated that coatings could soften and be impressed by support materials during drying (Figs 18, 19, and 20). Results from Variation (b) indicated that color and gloss could change (Figs 15 and 16, App. C). However. although all the papers underwent yellowing. the changes in color, reaching a maximum delta of 0.6 for the b* value of L*a b* for paper "B." are less than the 2 units required to be detectable by the human eye. Gloss measurements indicate that the glossiest paper "L," underwent the greatest (unit) reduction in gloss after treatment. According to the gloss measurements. immersion (I) generally appeared to cause greater reduction than poulticing (P), and air drying (AD) generally appeared to cause greater reduction than blotter drying (BD). With regard to the samples testing in this study, immersed air dried (IAD) papers generally underwent the greatest loss of gloss~ while poulticed blotter dried (PBD) samples generally underwent the least. on the other hand. SEM imaging indicated that the poulticed samples had more surface marring than the immersed samples (Fig. 20).
IV Conclusions
Taken in summary, the findings from these studies. in response to the initial questions stimulated by the treatment of the chromolithographic Valentine. indicate: 1) all treatments left visible changes on all paper samples when viewed by SEM imaging (Figs 17-20): 2) softening could occur, as seen in impressions made by the spun-woven polyester webs in study 2 (Figs 18 and 19); and 3) appearance measurements for study 3 indicate that generally, all papers appear to have darkened and yellowed after all treatments (although within the range of visual tolerance): and all papers showed a loss of gloss, with the greatest change generally in the Immersed air dried samples (IAD) and the least change generally h the poultice blotter dried (PBD).
V Acknowledgements
The authors would like to thank the many people who contributed in various ways to this project: Laurel Carroll, Spencer Crew, Ron Cunningham, Melanie Feather, John Fleckner. Paul Glenshaw, David Haberstich, Elizabeth Harris, Marycolette Hruskocy, Emily Klayman, Mark Leithauser, Nancie McRaney, Marion Mecklenburg? Daniel Nguyen, Scott Odell, Elissa O'Loughlin, Nancie Ravenel, Jeani Shapiro, Jane Smith, Don Williams, and Mel Wachowiac, and Helena Wright. Special thanks goes t´, Murray Lebwohl for donating the scrapbook referred to in this paper.
Figures
| Fig. 20a: SEM photomicrograph of surface of modern machine-coated sample L, immersed in deionized water and air dried totally, as outlined in App. B for Study 3, Variation (a). | Fig. 20c: SEM photomicrograph of surface of modern machine-coated sample L, poulticed with blotter saturated with deionized water, and then air dried totally, as outlined in Appn. B for Study 3. Variation (a). |

| 
|
| Fig. 20b: SEM photomicrograph of surface of modern machine-coated sample B, immersed in deionized water and dried under spun-woven polyester web, blotter, felts and plexiglass (less than 1 PSI) as outlined in App. B for Study 3. Variation (a). | Fig. 20d: SEM photomicrograph of surface of modern machine-coated sample L, poulticed with blotter saturated with deionized water, and then dried under spun-woven polyester web, blotter, felts and plexiglass (less than 1 PSI) as outlined in App. B for Study 3, Variation (a). Shows some cracking or lifting of surface. |

| 
|
VI Appendices
Appendix A: Composition of Pigment-Coated Papers
Study 1: the traditionally hand-coated papers were coated. modified from recipes in Watrous. with one of three groups of reagent grade binders and compounds' characterized in comparison to other common coating pigments in table 1 below.
| Groups. based on compounds. of hand-coated papers produced for this study: | |
| I | calcium carbonate:Liquitex rabbit skin glue (1:3)
applied in 5 coats calcium carbonate: gum arabic (1:2) |
| II | zinc oxide: Liquitex rabbit skin glue (2:3) applied
h, 5 coats zinc oxide:gum arabic (2:3) |
| III | zinc oxide:Liquitex gel acrylic medium barium sulfate and calcium carbonate: Liquitex gel acrylic medium |
Table 1: Elemental characteristics of common coating pigments:
| PIGMENTS | Al | Si | K | Ti | Ca | S | Ba | Zn | n* | ìm** |
| blanc fixe (barium sulfate) | - | - | - | - | - | + | + | - | 1.65 | 0.11-0.54 |
| cal. carbonate | - | - | - | - | + | - | - | - | 1.66 | 0.1-10 |
| clay | + | + | -/+ | (+) | - | - | - | - | 1.55 | 2 |
| gypsum | - | - | + | + | - | - | 1.52 | |||
| satin white | + | - | - | - | + | + | - | - | 1.46 | 1-2X.1.2 |
| talc | (+) | - | - | - | (+) | - | - | - | 1.57 | 2 |
| titanium dioxide | - | - | - | + | - | - | - | - | 2.5-2.7 | 0.2-0.5 |
| zinc oxide | - | - | - | - | - | - | - | + | 2.01-2.03 | 0.3-0.5 |
(*n=refractive index, ** ìm**=micron)
Study 2: The authentic glazed chromolithographs came from a donated scrapbook. Samples were selected on the basis of their similarity to the Victorian card undergoing treatment. SEM/EDS of powdered coating samples from each indicated three basic groups. Each sample is listed with a brief description. page number from the scrapbook. and elemental composition. in table 2 below. Groups, based on compounds, of authentic chromolithographs used in this study:
| I | primarily clay (Al, Si) and barium sulfate (Ba, S) |
| II | primarily clay (Al, Si. K) and barium sulfate (Ba, S) |
| III | primarily clay (Al, Si, K) and barium sulfate (Ba, S) and gypsum, talc, or satin white (Ca) |
Table 2: Elemental composition of powdered pigment-coatings of authentic chromolithographs:
| CHROMOLITHOGRAPHS | Al | Si | K | Ti | Ca | S | Ba |
| I Red flower (p. 11) | + | + | - | - | - | + | + |
| Flower bunch (p.11) | + | + | - | - | - | + | + |
| Everthine (p.11) | + | + | - | - | - | + | + |
| To my dear (p. 11) | + | + | - | - | - | + | + |
| Woman (p. 5) | + | + | - | - | - | + | + |
| II Vict. Valentine card | + | + | + | - | - | + | + |
| Friendship card | + | + | + | - | - | + | + |
| Flowers (p.19) | + | + | + | - | + | + | + |
| Child (p. 21) | + | + | + | - | + | + | + |
| (Modern Repro.) | + | + | + | + | - | - |
Study 3: The modern machine-coated papers were supplied by various manufacturers. win., described them as follows:
| "B": | Machine-made in France as "Special Point d'Argent: Calligraphie" (recommended by manufacturer for silver point drawings) |
| "C": | Machine-made 100% rag paper with neutral pH. Smooth. clay-coated: recommended for silverpoint drawing. |
| "F": | Machine-made glossy, light weight stock, available in 28 colors. |
| "K": | Glossy white text weight paper recommended for illustrations and ink drawings. |
| "L": | Machine-made in Germany; smooth glossy surface on 2 ply 10 point card stock. |
| "Q": | Machine-made with fine clay coating. Ivory color. recommended for silverpoint drawing. |
| "R": | Machine-made 100% rag paper with neutral pH. Smooth. clay-coated (2 sided): glossy on one side and matte on the other. Recommended for offset printing but not silverpoint drawing. |
| "S": | Machine-made paper with smooth clay coating recommenced for silverpoint drawing. |
The papers fall into three basic groups as indicated by SEM/EDS analysis in table 3 below.
Groups based on compounds of modern pigment-coated papers used in this study:
| I | primarily barium sulfate (Ba, S) and talc (Si, Ca) |
| II | primarily clay (Al Si K) and gypsum (Ca S) |
| III | III primarily clay (Al Si) and talc (Ti Ca) or titanium dioxide and alcium carbonate |
Table 3: Elemental composition of modern coated papers:
| MODERN COAT. | Al | Si | K | Ti | Ca | S | Ba |
| "B" | - | + | - | - | + | + | + |
| "C": | + | + | + | - | + | + | - |
| "F" | + | + | + | - | + | + | - |
| "K" | + | + | - | + | - | - | - |
| "L" | + | + | - | + | + | - | - |
| "Q" | + | + | - | + | + | - | - |
| "R" | + | + | - | + | + | - | - |
| "S" | + | + | - | + | + | - | - |
Appendix B: Treatment Procedures
Study 1: Effects of solvents and application techniques on traditionally hand-coated papers:
Immersion: for immersion the end of each sample was dipped into a 3 mL deep soh~tion ´,fs´,lvent tl~r 3 seconds and then air-dried.
Poultice: the solvent poultice consisted of diatomaceous earth saturated by solvent (approximately 1-2 ml solvent to 0.3 grams diatomaceous earth depending on solvent) and placed on the sample. Contrary to normal practice the wet poultice was not surrounded by dry poultice to diffuse the transition from wet to dry areas.
Diatomaceous earth (hydrated silica from diatom plant skeletons) was selected for its working properties since unlike gel poultices (methylcellulose agarose starch paste. or hydroxypropylethylcellulose) it can be mixed with aqueous or non-aqueous solvents to form a plaster or paste that absorbs solutes as it dries to a powder which can then be brushed oft. It is more cohesive than fused silica. It is whiter than Fuller's earth which is formed from hydrated silicates of magnesium. calcium. aluminum. or other metals. It is more controllable than organic solid poultices such as powdered cellulose, paper, or cotton.
Suction disk: Solvents were applied locally by dropper on a 15 cm fritted glass head disk (masked off with polyester film) which can reach a pressure of. 25" Hg.
Study 2: Effects of water on glazed chromolithographs:
Deionized water was brushed onto the surface of the samples. Excess water was absorbed by a blotter.
Study 3: Effects of water on modern machine-coated papers:
(a) Variation (used samples 1 cm square; spun-woven, fibrous polyester; unbuffered blotters):
Immersion Procedure: the samples were immersed in deionized water on a spun-woven fibrous polyester web. the samples remained immersed for five minutes. the web and samples were lifted out of the water and allowed to drain for two minutes. Half of the samples were lifted on the polyester web to a blotter on a felt and were allowed to air dry totally. Appearance measurements (color and gloss) were taken after two days.
The other half of the samples were placed onto a blotter on a felt r 5-10 minutes until all standing water on the surfaces of the papers evaporated. The blotter under the samples was replaced by a dry blotter and these samples were then covered with polyester web. blotter. and felt. and were blotted with hand pressure. Both top and bottom blotters were changed and the ensemble was placed for five minutes in a "press" under a felt and 1/2 inch thick piece of plexiglass (less than 1 PSI). Changes were made of the blotter after 1 hour and of the polyester web and blotter after 1 day. Appearance measurements (color and gloss) were taken after two days.
Poultice Procedure: The blotter used for poulticing was dampened by immersing it in a tub of deionized water. The blotter was drained and allowed to sit for five minutes to enable standing water to evaporate for more even moisture distribution. The blotter was then flipped over onto the samples which were against a formica countertop for 5 minutes. Afterwards the clamp blotter was removed~ the wet samples were exposed to the air for two to five minutes. until all standing water on the surfaces of the papers evaporated. Halt of the samples were left to air dry totally. Appearance measurements were taken after two days.
The other half were moved to a blotter "press" made up from the bottom up, of the formica counter, felt. blotter, polyester web, sample, polyester web, blotter, felt, plexiglass (less than 1 PSI). The samples were left overnight in the press. the next day the polyester web and blotter were changed and the ensemble was returned to the "press." Appearance measurements were taken after two days.
(b) Variation (used samples 8" X 10"; non-woven smooth polyester web. unbuffered blotters):
Immersion Procedure: The samples were immersed in deionized water on a polyester web (nonwoven. with a smooth surface. 5 mil) on a polypropylene screen. The samples remained immersed for five minutes. The screen with web and samples was lifted out of the water and allowed to drain for two minutes. Half of the samples were lifted on the polyester web to a blotter on a felt and were allowed to air dry totally. Appearance measurements (color and gloss) were taken after two days.
The other half of the samples were placed onto a blotter on a felt and allowed to air dry for 5-10 minutes until all standing water on the surfaces of the papers evaporated. The blotter under the samples was replaced by a dry blotter and these samples were then covered with polyester web, blotter, and felt and were blotted with hand pressure. Both top and bottom blotters were changed and the ensemble was placed for five minutes in a "press" under a felt and 1/2 inch thick piece of plexiglass (less than 1 PSI). The blotters were changed again after one hour and returned to the "press". The polyweb and blotter were changed the next day and returned to the "press." Appearance measurements (color and gloss) were taken after two days.
Poultice Procedure: The blotter used for poulticing was dampened by Immersing it on a screen in a tub of deionized water. The screen was removed and the blotter was drained on the screen for two minutes. A piece of 4 mil polyester film was placed on the blotter resting on the screen and then the blotter was flipped over so that the polyester film was on the bottom. The blotter was allowed to sit for five minutes to enable standing water to evaporate for more even moisture distribution. The blotter was then flipped over onto the samples which were against a formica countertop and the polyester film was removed. A felt and a 1/2 inch thick piece of plexiglass were place on the blotter poultice for 5 minutes. After the plexiglass felt and damp blotter were removed, the wet samples were exposed to the air for two to five minutes, until all standing water on the surfaces of the papers evaporated. Half of the samples were left to air dry totally. Appearance measurements were taken after two days.
The other half were moved to a blotter "press" made up. from the bottom up. of the formica counter, felt blotter polyester web, sample, polyester web blotter, felt, plexiglass (less then 1 PSI). The samples were left overnight in the press. the next day the polyester web and blotter were changed and the ensemble was returned to the "press." Appearance measurements were taken after two clays.
Appendix C: Experimental Design for Study 3, Variation (B) .
| RESEARCH DESIGN | ||||||||||||||
| 1. Select Sample Chromolithographic Papers | ||||||||||||||
| 2. Identify Components by Elemental Analysis | ||||||||||||||
| 3. Characterize Samples by Measuring Appearance Properties | ||||||||||||||
| 4. Treatment (Aqueous) | Untreated | |||||||||||||
| Immersion | Poultice | Control | ||||||||||||
| A1 | A2 | A3 | B1 | B2 | B3 | C1 | C2 | C2 | ||||||
| Air | Blot | Air | Blot | Air | Blot | Air | Blot | Air | Blot | Air | Blot | |||
| 6. Test for Changes in Elemental
Composition Morphology, and Appearance Properties (Note: each sample Al-C3 for each paper type was tested 3 times) | ||||||||||||||
Experimental Conditions and Labelling:
(Each treatment trial was conducted three times and each resultant sample was measured three times for statistical accuracy)
| MODERN PIGMENT COATINGS | S | L | B |
|---|---|---|---|
| Control (C) | 1=C | 14=C | 27=C |
| Immerse/airdry (IAD) | 2=IAD. I (3 meas) | 15=IAD.1 (3 meas) | 28=IAD.1 (3 meas) |
| 3=IAD.2 (3 meas) | 16=lAD.2 (3 meas) | 29=IAD.2 (3 meas) | |
| 4=IAD.3 (3 meas) | 17=lAD.3 (3 meas) | 30=IAD.3 (3 mcas) | |
| Immerse/blotter (IBD) | 5=IBD. 1 " | 18=IBD.I " | 31=IBD.1 " |
| 6=IBD.2 " | 19=IBD.2 " | 32=IBD.2 " | |
| 7=IBD.3 " | 20=IBD.3 " | 33=IBD.3 " | |
| Poultice/airdry (PAD) | 8=PAD.1 " | 21=PAD.1 " | 34=PAD.1 " |
| 9=PAD.2 " | 22=PAD.3 " | 35=PAD.2 " | |
| 10=PAD.3 " | 23=PAD.3 " | 36=PAD.3 " | |
| Poultice/blotter (PBD) | 11=PBD.1 " | 24=PBD.1 " | 37=PBD.1 " |
| 12=PBD.2 " | 25=PBD.2 " | 38=PBD.2 " | |
| 13=PBD.3 " | 26=PBD.3 " | 39=PBD.3 " |
Data: Gloss 85 degrees (Standard Deviations)
| ABSOLUTE: | S | L | B |
| Control (C) | 1=11.8 +/-0.4 | 14=96.9 +/-0.3 | 27=18 +/-0.2 |
| DELTA: | |||
| Immerse/airdry (IAD) | 2-4=6.2 +/-03 | 15-17=31.4 +/-2.5 | 28-30=5.4 +/-1.2 |
| Immerse/blotter (IBD) | 5-7=5.8 +/-0.3 | 18-20=27.9 +/-1.0 | 31-33=3.7 +/-0.4 |
| Poultice/airdry (PAD) | 8-10=5.3 +/-0.5 | 21-23=30.4 +/-13.8 | 34-36=5.2 +/-0.9 |
| Poultice/blotter (PBD) | 11-13=4.1 +/-0.7 | 24-26=22.2 +/-6.2 | 37-39=3.5 +/-0.4 |
Appendix D: Analytical Procedures
SEM: SEM imaging and SEM/EDS analysis were carried out on a Jeol JXA - 840 a scanning electron microscope with Tracore Northern TN 5502 energy dispersive x-ray analysis system. F<,r imaging the samples were mounted on aluminum stubs and gold coated. for elemental analysis the samples were mounted on carbon stubs and carbon coated.
FTIR: FTIR analysis was carried out on a Mattson Cygnus 100 Fourier Transform Infrared Spectrophotometer with a Spectratech IR-Plan Microscope. Surfaces were analyzed by reflectance, or alternately micro samples were removed and pressed into thin films in a diamond anvil cell for analysis by transmission.
Colorimetry: Color (specular reflectance included) was measured with the HunterLab Ultrascan Spectrocolorimeter (D65, 10º observer, diameter of area of view 1.2 in) using the CIE L*a*b* color notation. where L* represents the degree of brightness (100 white. 0 black). a* the degree of redness (positive numbers) or the degree of greenness (negative numbers) and b* the degree of yellowness (positive numbers) or the degree of blueness (negative numbers). Three measurements were taken per sample and averaged.
Gloss: Gloss was measured with a Dr. Lange Labor-Retlektometer RL at angles: 20'. 60' and 85'. Three measurements tor each angle were taken per sample and averaged.
VII. Selected Bibliography
1. Alien~ Alistair. The History of Printed Scraps. London: New Cavendish. 1983.
2. Allen, Alistair-. Scraps-150 Years of Stamped. Embossed Reliefs. Bethnal Green Museum of Childhood, Exhibition Feb-April 1977. England, Wisbech: Balding, and Mansell. 1977.
3. Baker, Mary, Dianne van der Reyden, and Nancie Ravenel. "FTIR Analysis of Coated Papers. The Book and Paper Group Annual, 8 (1989), pp 1-12.
4. Brunner, Felix. A Handbook of Graphic Reproduction Processes. Teufen AR. Switzerlancl: Arthur Niggli Ltd., 1975.
5. Buday, George. The History of the Christmas Card. London: Spring Books. 1954.
6. Buist, J.S. "The Magic of Scraps." The Slots Magazine. New Series Vol. 106. No. 3. Dec. 1976, pp. 237-243.
7. Casey, James P. Pigment-Coated Papers a Critical Assessment of the Processes, Technical Developments. and Economics. New York: Marcel Dekker, Inc.. 1985.
8. Champion International Corp. Imagination 26: White on White: New Champion Kromekote 2000. Stamford, Conn. c. 1988.
9. "Color-Plates for Chromolithography, " Lithographer's Journal, July 1893, p. 8.
10. Denstman, Harold and Morton J. Schultz. Photographic Reproduction. New York: McGraw Hill Book Company. Inc., 1963.
11. Droscher, Elke. The Victorian Sticker Postcardbook. Philadelphia: Running Press. 1982.
12. Ellis, Margaret Holben. "Metalpoint Drawings: Special Conservation Problems for Collectors." Drawing, II, 3, 1980. pp. 59-61.
13. Etter, Roberta B. Tokens of Love. New York: Abbeville Press. 1990.
14. Fleischman. John. "The labyrinthine world of the Scrapbook King." Smithsonian. Feb. 1999. pp. 78-87.
15. Fournier, Raymond. "Barium and the Paper Industry," La Papeterie, Vol. 55, No. 13 (1933). pp. 666, 669-670, No. 14, pp. 722-726.
16. Gascoigne, Bamber. How to Identify Prints. New York: Thames and Hudson, 1986.
17. Hackleman, Charles W. Commercial Engraving and Printing. Indianapolis: Commercial Engraving Publishing Company, 1921.
18. Harding, Eric. "Restoration of drawings executed on prepared paper." Conservator, I (1983). pp. 24-28.
19. Hart. Cynthia and John Grossman. A Victorian Scrapbook. New York: Workman Publishing, 1989.
20. Hart. Cynthia and John Grossman. Forget Me Nots. New York: Workman Publishing. 1990.
21. Janse, William M. The History of Valentines. Chatham, Mass: William M. Janse. 1950
22. Kampmann, C. "Substitute for Lithographic Stone," Lithographer's Journal. June 1893. p. 10.
23. Kirsch, Francine. Chromos-A Guide to Paper Collectibles. San Diego. New York: A.S. Barnes & Company, Inc., 1981.
24. Kouris. Michael and Dr. Michael J. Kocurek. Pulp and Paper Manufacture, Vol. 8 Coating. Converting, and Specialty Processes. Ste-Anne-de-Bellevue. Que.: The Joint Textbook Committee of the Paper Industry. Harpell's Press Co-operative, 1990.
25. Lambert, Luna Frances. The Seasonal Trade: Gift Cards and Chromolithography in America. A dissertation submitted to the graduate school of arts and sciences of the George Washington University in partial satisfaction of the requirements for the degree of Doctor of Philosophy. 1980, 191p.
26. Le Blon, J.C. Coloritto (Facsimile Edition). New York: Van Nostrand Reinhold Company 1980
27. Lee, Ruth Webb. A History of Valentines. Wellesley Hills, MA: Lee Publications. 1952.
28. Maclead, Martin. "Early History of Coated Papers-How They Came of Age." Paper Trade Journal, May 27, 1972, pp. 170- 175.
29. Marchessault, R.H. and Christen Skaar. Surfaces and Coatings Related to Paper and Wood. Syracuse: Syracuse University Press, 1967.
30. Marzio, Peter C. The Democratic Art. Boston: David R. Godine, 1979.
31. Mortimer, Cromwell. "An Account of Mr. James Christopher Le Blon's Principles of Printing. in Imitation of Painting, and of Weaving Tapestry. in the same manner as Brocades. Philosophical transactions of the Royal Society. No. 419 (June and July 1731), pp. 101-107.
32. Murray, Hayden H., ed. Paper Coating, Pigments-TAPPI Monograph Series No. 30. 1966. New York.
33. Pieske, Christa. Das ABC des Luxuspapiers: Herstell~m~. Verarbeitung und Gebrauch 1860 bis 1930. Staatliche Museen Preussischer Kulturbesitz, Berlin, 1983.
34. Prang, Louis. Prang's Chromo, A Journal of Popular Art, Vol. 1, No. 1. January. 1868.
35. Prang, Louis. Prang's Chromo, A Journal of Popular Art, Vol. 1, No. 4, Christmas 1868.
36. Richmond, W.D. Colour and Colour Printing as Applied to Lithography. London: Wyman & Sons, no date.
37. Richmond, W.D. The Grammar of Lithography. London: Meyers & Co., 1914.
38. Rickards, Maurice. Collecting Printed Ephemera. New York: Abbeville Press. 1988.
39. Schuart, Harry W. " Decorative scraps for Christmas", The Spinning Wheel, Dec. 1971 ~ 1~ 8- 10.
40. Singer, Dr. Hans W. "Jakobh Christoffel Le Blon and His 3-Colour Prints." The Studio. 28 (1903). pp. 261 -271.
41. Staff, Frank. The Valentine and Its Origins. New York: Praeger, 1969.
42. TAPPI. Pigment Coating Processes-TAPPI Monograph Series-No. 28. New York: Technical Association of the Pulp and Paper Industry, 1964.
43. TAPPI. Pigments for Paper Coating -TAPPI Monograph Series-No .7 New York: Technical Association of the Pulp and Paper Industry, 1948.
44. Twymann, Michael. Lithography 1800-1850. London: Oxford University Press. 1970.
45. West. Clarence J. Coating of Printing Papers Bibliographic Series Number 162. 2nd ed. Appleton, WI: IPC, 1952.
46. West, Clarence J. Bibliography of Pulp and Paper Making 1900-1928. New York: Lockwood Trade Journal Co.. Inc., 1929.
47. Van der Reyden, Dianne, Erika Mosier. and Mary Baker. "Pigment Coated Papers: the Effects of Some Solvent Application Techniques on Selected Examples." submitted to Working Group for Conservation of Graphic Materials. ICOM. for Washington DC Meeting,. August. 1993.
48. Volent. Paula. "The Conservation Treatment of an Oversize Drawing by Ann McCoy." WAAC Newsletter. Vol. 10. No. 3 (Sept. 1988).
49. Watrous, J. The Craft of Old Master Drawings, University of Wisconsin Press, 1957.
Notes
2. 1890 marked an increased use of baryta or precipitated barium sulfate from barite. Coprecipitation 7:3 with zinc sulfide forms lithopone which became a substitute for lead carbonate. Barium sulfate comes in two forms: natural mineral barite or artificial blanc fixe which may be derived from baryte or witherite (a barium carbonate ore in England and Europe). Baryta or blanc fixe makes a brighter denser and less porous coating used at the end of the century for photographs and special grades of chart papers (J.P. Casey Pulp and Pacer: Chemistry and Chemical Technology 3. 2nd ed. (New York: Intersciences Publishers 1961).
3. 1%. agarose (Sigma Type VII No. A-4018) poultice on lens tissue under polyester film (Mylar Brand)
4. .01% Calbiochem Pronase 53702 in tap water
5. FTIR identified the glaze (over a section of the chromolithograph with metallic brass colored ink) as cellulose nitrate and an unglazed section of pigment coating as having a proteinaceous binder (3).
Erika MosierDianne van der Reyden
Mary Baker
Conservation Analytical Laboratory
Smithsonian Institution
Publication History
Received: Fall 1992
Paper delivered at the Book and Paper specialty group session, AIC 20th Annual Meeting, June 2-7, 1992, Buffalo, NY.
Papers for the specialty group session are selected by committee, based on abstracts and there has been no further peer review. Papers are received by the compiler in the Fall following the meeting and the author is welcome to make revisions, minor or major.