Tests on the Effects of the Use of Ultrasound in the Humidification of Paper
by Niccolo CaldararoAbstract
This paper discusses the implications of sonochemical research on the use of ultrasound in the delivery of moisture to paper objects.
Introduction
The use of ultrasound in cleaning objects of inorganic and organic composition has become widely practiced in commercial enterprises as well as in the examination of objects for flaws in structural integrity (1-5). Since its introduction into the conservation literature by Organ in 1959 (6) ultrasound has had frequent application to a wide range of materials (7-13). The source of the cleaning action is the delivery of mechanical waves produced by the piezoelectric effect through a gas or liquid medium to the surface of the object. Basically, this is the phenomenon caused by the application of oscillatory electrical energy on two metal plates creating an electrical field in which a crystal vibrates generating waves in a medium (1-5). This process in reverse is exploited in microphones where sound waves are transferred from the air to a crystal of piezoelectric material and thereby made available for amplification.
Background
A review of the use of ultrasound in conservation and related fields can put this technique into perspective since its application is spreading within the discipline (14,15,26,29).
The two delivery media in ultrasonic use, gas or liquid, have lent themselves to various commercial applications and in conservation, as a hand-held vibro-tool (an adaptation of common dental equipment) and in baths to which ultrasonic waves are exposed.
In his article, Organ (6) cautioned readers against potentially damaging effects of ultrasound (17). Firth (18) elaborated on these dangers with respect to cleaning fossils. As a scientist Organ was particularly aware of the chemical activation which could result from any exposure to ultrasound. Organ's described application was to cleaning bronze, materials which he could be reasonably sure would not be greatly affected by the energy delivered by ultrasonic waves (19). Later applications of ultrasound have been made to a wide variety of materials both organic and inorganic (7-13,14,16,18,20,21). A recent article by Abdulla (22),and three by Suslick and Doktycz (39, 40), however, should strike a note of caution to any conservator who has applied or contemplates the application of ultrasound to materials in collections, especially organic materials.
One must keep in mind that ultrasound, generally the subject of sonochemistry, is a potent form of non-electromagnetic radiation. This radiation, like other forms of radiation, can start some and accelerate other chemical reactions (22). The results of ultrasonic radiation depend upon the type of application. Some examples are, a) simple exposure with an ultrasonic dental cleaner or scaler, such as the Cavitron tool, b) long duration of exposure, c) variation introduced by the solvent or gaseous environment, d) chemical interactions introduced with (c) above, by the constituent materials of an object and its structure. For conservation purposes some examples of change are notable: yellow HgI2 is converted to red HgI2 (actually a phase change) at temperatures below 120 degrees centigrade (see Abdullah 22 and 40), compare with Plester, et al, (23), and small glass fragments can be 'abraded' from the walls of a test tube filled with distilled water (22). Chemical reactions are not only accelerated but some are initiated which do not occur under normal heating or mixing of reagents (22). The main processes of ultrasonic action is "cavitation" often mix-defined as the creation of vacuum bubbles. Actually, the process of cavitation is a complex process involving the formation of bubbles in the solution used into which gases diffuse, and followed by adiabatic compression. Though the exact action in cleaning or chemical synthesis is not known in all cases, the simple mechanical action is probably one of several agencies responsible for the cleaning observed.
We must realize that exposure to ultrasound is a process more complex than a visualized applying of vibrations. Grattan (24) has kindly reminded me in this regard that, with reference to the kinetic theory, energy applied as one form of thermal energy (vibrational, translational or rotational) is redistributed to the others (25). We must consider rate changes and activation energies whenever we apply energy to objects. Ultrasound is, in this regard, initiating a more intense reaction potential on component materials than the simple application of heat (41). Reactions, therefore, follow the ultrasonic irradiation of liquids (26) which can have profound effects on immersed objects, as when in water hydrogen peroxide is formed which can result in bleaching (27). Rath and Merk report fiber damage under certain circumstances (28). Of course a knowledge of the chemical and physical effects of ultrasound can be used to great advantage if conditions can be controlled to produce desired results as Masuda (29) has reported combining the suction table and ultrasound. One must, however, still weigh these results against other changes initiated by ultrasound.
Little experimental work has been done on the effects of ultrasound on organic objects. The work of Katz and Man (30) has shown that ultrasound radiation results in the introduction of significant diagenic changes in shell. For polymers in general, Russian investigators have found that exposure of polymethyl methacrylate results in degradation of the polymer chain (31). More work in this area needs to be done combining experimental work with treatment analysis. However, future studies should test several other modalities of treatment with ultrasound as well as with different types of materials of which objects are composed.
Tests
Other effects of ultrasound versus other methods can be easily observed by microscopy, for example, my own experiments with paper comparing typed text onto Strathmore 20% cotton fiber typewriter stock with text applied by a Brother Ax-22 electronic typewriter from a Brother correctable cartridge, with sample exposed to:
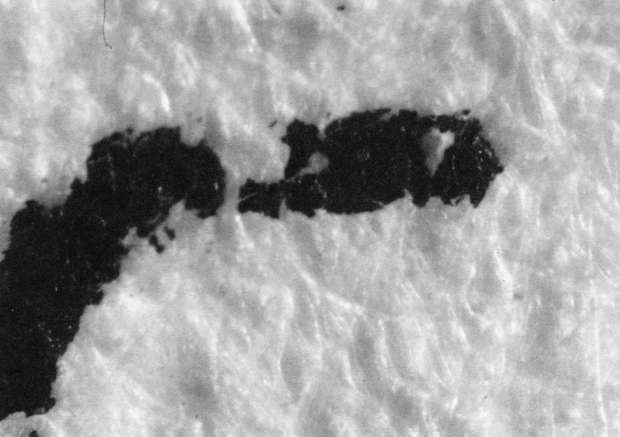
- Soaking only in de-ionized water for 10 minutes(see illustration "B").
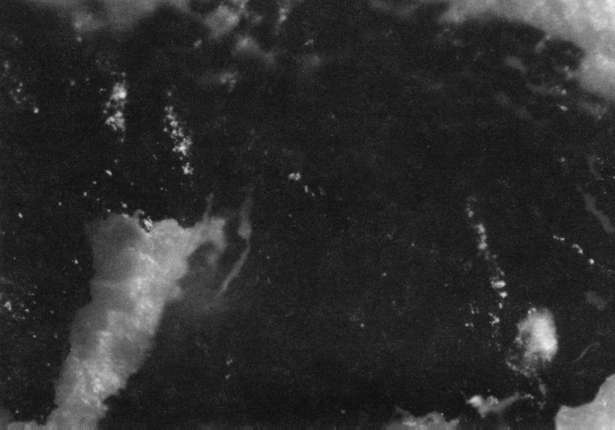
- Exposure to steam from an Osrow Model SB hand fabric steamer from 3 to 5 minutes, basically enough time to saturate the paper with moisture (see illustration "C"). The paper sample was taped to a piece of rag board and fixed in a slightly inclined position as is usual when using a steamer to deliver moisture during such standard operations as backing removals (42). The steamer mouth was kept at least 3 to 5 inches from the sample to minimize heating.
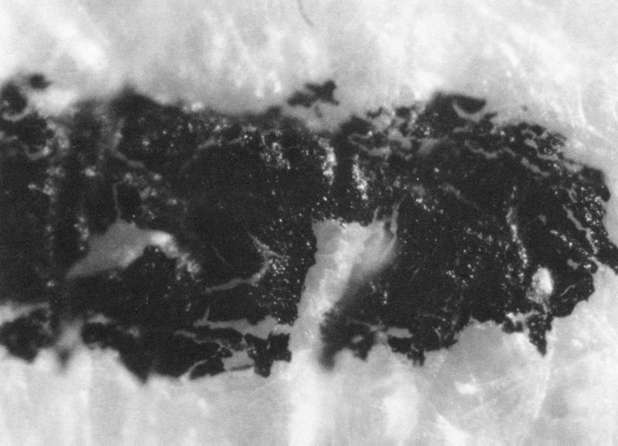
- Exposure to an ultrasonic bath in de-ionized water for 5 minutes in Sonicor Model SC-105T, 50/60Hz cycles, 1.5 amps (see illustration "E"), and
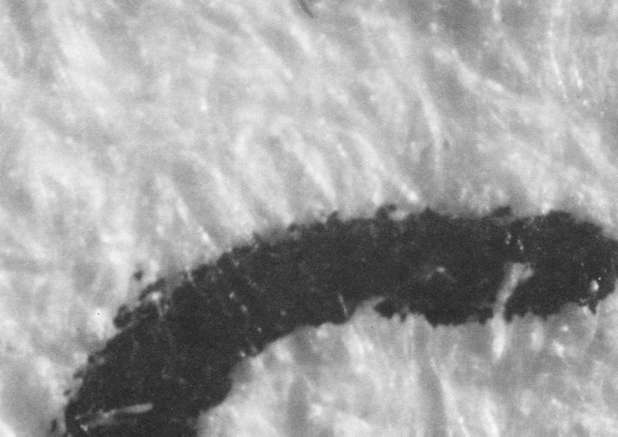
- Exposure to moisture delivered from an ultrasonic humidifier, a Biotech BT-200 unit, for 5 minutes(see illustration "D").
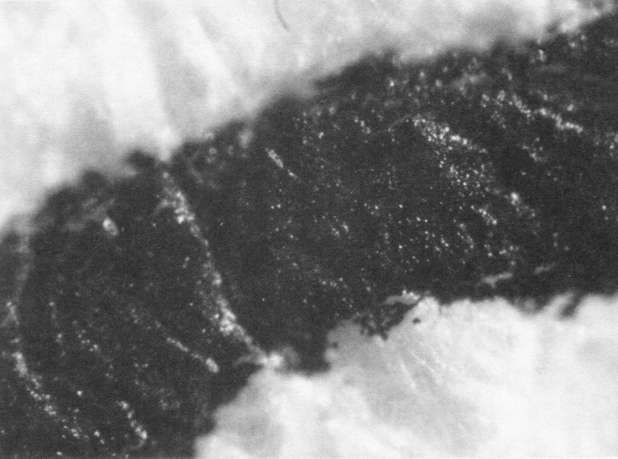
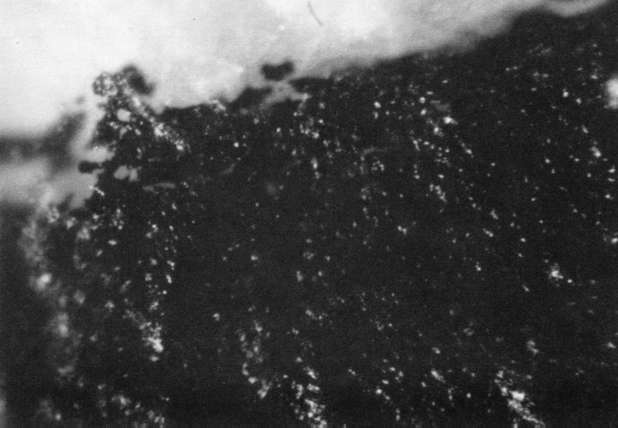
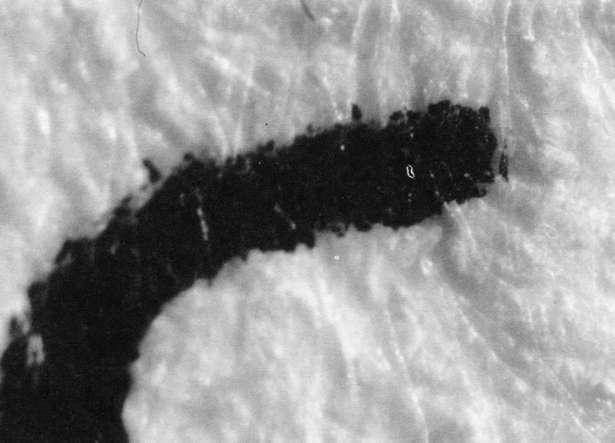
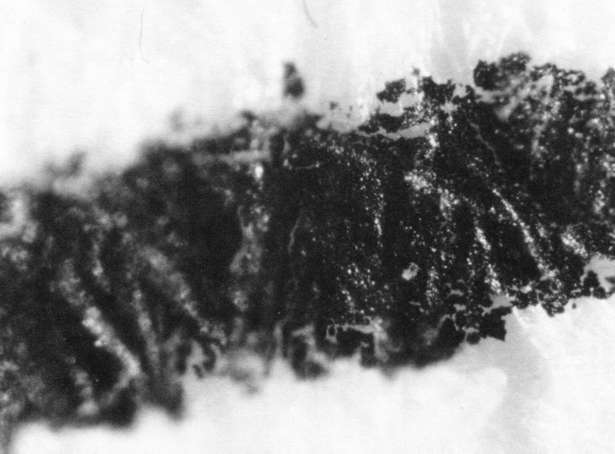
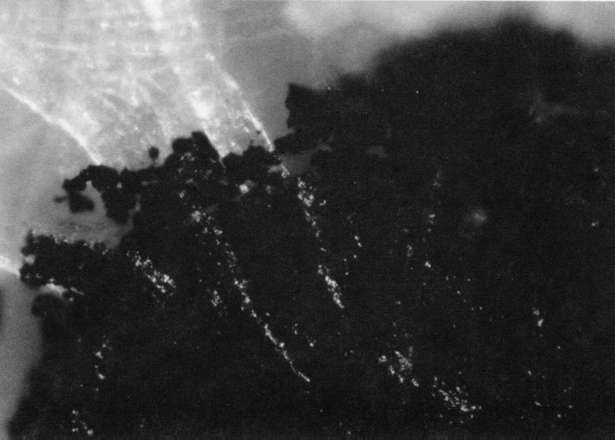
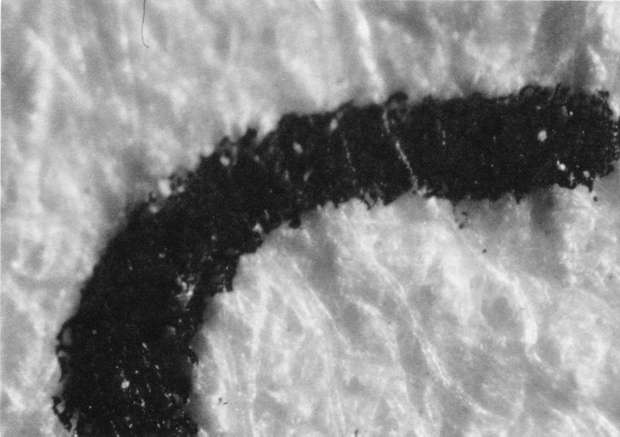
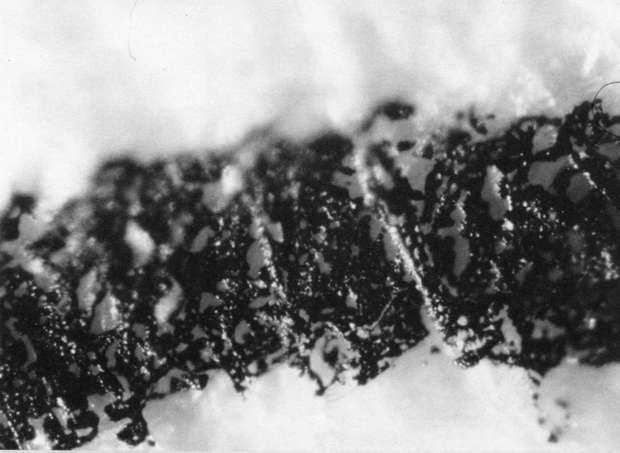
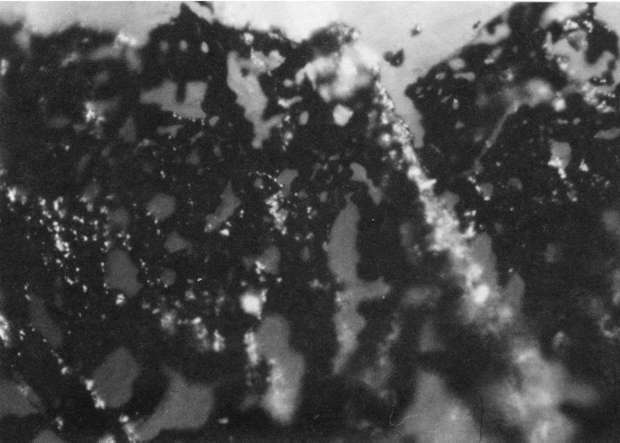
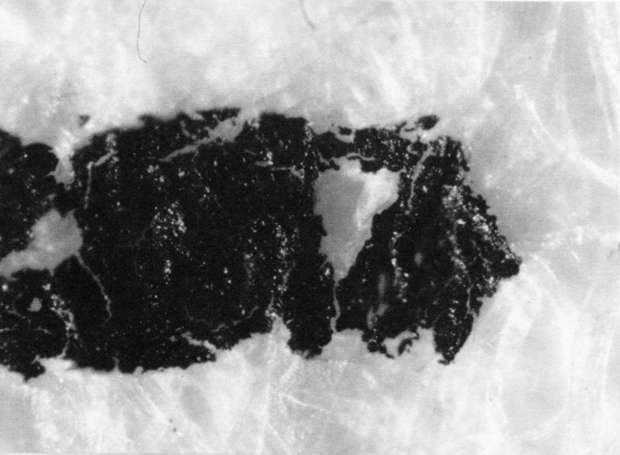
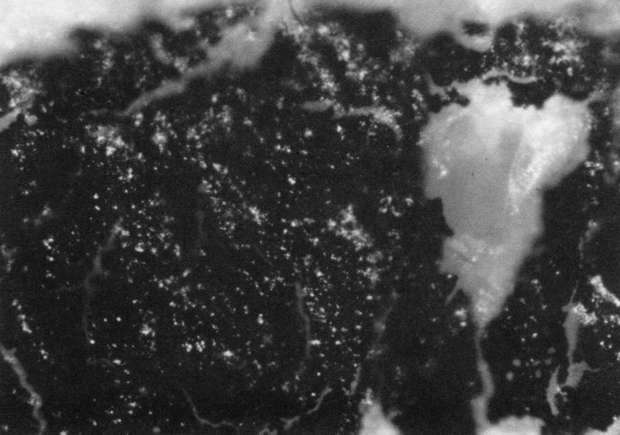
Comparison of these results with the control (illustration "A") showed that the letter matrix fragmented and fissured in the ultrasonic bath and less so under the direction of moisture delivered by ultrasonic humidifiers. Fiber movement is evident in all the treatment samples compared with the control sample, even the water only bath (see "A" & "B"). The sample exposed to steam ("C") showed a deterioration of the type matrix which appeared to be due to melting or solvent action. But soaking samples in hot water (125° F) did not produce the same effect, but samples placed in an oven at 175° C exhibits the same kind of effect. The samples exposed to ultrasound humidification generally appeared like those bathed only in water, however, the checking of the type matrix appears strikingly similar in both "D" & "E", and while the energy delivered by humidification cannot be great, there may be some affinity between the matrix components and that energy. Further study of the uses of ultrasound in conservation should be undertaken to identify these relationships, especially in those applied to paper objects(5).
Studies like that of Smith, et al (32) with pressure-sensitive tapes provide a wide range of physical and chemical approaches to the same problem and thereby a clear means of modality comparison. Of course, the lack of illustrations to show the effects of treatments or of standards to indicate acceptable (or successful) levels of removals (fiber disturbance versus skinning, etc.) is unfortunate. For example, how do we judge how much adhesive residue left in the paper support is acceptable (33,34), or how much abrasion to the paper support? Questions like these must be answered for the field of conservation to make progress as a scientific discipline (35).
Rose, et al, (36) have produced a very useful study that compares numerous types of treatment modalities (erasers) with one example of another modality (feather dusters). The effects of using both are amply illustrated in showing the results of their application by excellent photomicrographs. Pearlstein, et al, have compared the effects of various erasers on the physical and chemical properties of paper (37). This was a valuable study and coupled with ones exploring the surface effects and visual results (as in Rose, et al, above) would complete the necessary data for a thorough evaluation. Hopefully, experimental work can be combined by future researchers with the use of comparative treatment effects using macro and microphotography illustrations to give us clear and practically oriented conclusions to studies (38).
Illustrations
A. Controls. Top 40x
A. Controls. Middle 100x
A. Controls. Bottom 200x
B. Samples bathed only in de-ionized water: 40x
B. Samples bathed only in de-ionized water: 100x
B. Samples bathed only in de-ionized water: 200x
C. Steam Only: 40x
C. Steam Only: 100x
C. Steam Only: 200x
D. Humidification via ultrasound: 40x
D. Humidification via ultrasound: 100x
D. Humidification via ultrasound: 200x
E. Samples in ultrasound bath: 40x
E. Samples in ultrasound bath: 100x
E. Samples in ultrasound bath: 200x
References
1. Naodi, F., Y. Mitsuishi, T. Hayashi and H. Okutama. 1985. Spotting of Water-Soluble Stains on Japanese Silk Kimonos. Part 2: The Removal Effect of Stains on Colorfastness of Kimonos by Drycleaning. J. Japan Research Association for Textile End-Users 26(7): 302-310.
2. Senko, E. and J. Thorpe. 1985. On-Line Ultrasonic Measurement of Sheet Modulus. Tappi J. 68 (2): 95-9.
3. Mazurek, J. 1985. Method of Verification of Plaster and Substrate Bonding. Pol. Acad. Sci Oct.
4. Mazurek, J. 1985. A Method of Evaluating the Compression Strength in Plaster with Ultrasonic Waves. Pol. Acad. Sci. Oct.
5. Curtin, Bonnie. 1988. Effect on Paper pH and Alkaline Reserve from Magnesium Bicarbonate Introduced via Ultrasonic Humidification. Preprints AIC; 1988: 276. See also, Timothy D. Barrett, "Early European papers/contemporary conservation papers: a report on research undertaken from fall 1984 to fall 1987", in The Paper Conservator, v. 13, 1989(section 2.6 "ultrasonic properties):49-53.
6. Organ, R. 1959. Treatment Using Ultrasonic Vibrations. Studies in Conservation 4: 35-8.
7. Kramer, W. 1964. In Restaurierung und Konservierung Verlag Bruno Hessling, Berlin.
8. Hempel, K.F.B. 1969. "The restoration of two marble statues by Antonius Corrudius", Studies in Conservation 14: 126. Also, Lazzarini, L., 1981. La Oulitura dei Materiali Lapidea da Construzione e Scultura - Metodi Industriali e de Restauro (The Cleaning of Stone Material in Building and Sculpture - Industrial Methods of Restoration), CED AM - Casa Editrice Dott. Antonio, Milani.
9. Goubitz, Olaf. 1982. The Cleaning of Archaeological Objects with an Ultrasonic Probe. Conservation News (UKIC Newsletter) 18: 8- 10. See also Hall, E.T., "Some uses of Physics in Archaeology", in Proceedings of a Seminar on Applications of Science in Examinations of Works of Art, September 15-18, 1958, Museum of Fine Arts, Boston(1959:181-193).
10. Lewis, Brian. 1981. A Preliminary Report on the Relative Effectiveness of ultrasonic Cleaning Versus Soaking in the Conservation of Clay Tablets. Iraq 43(1): 76-8. See also Spence, K., "Keeping a Cathedral Alive", Country Life, CLVIII(4070), 1975:6-8.
11. Molkenboer, J.A. 1980. Ultrasonic Cleaning and Degreasing. Metalloberflaeche 34(4): 159-162. 12. Muhlethaler, B. 1973. Conservation of Waterlogged Wood and Wet Leather, Paris, Pg. 67. See Also, van Dienst, E., "Some remarks on the Conservation of Wet Archaeological Leather", Studies in Conservation, 30, 1985:86-92.
13. Chase, W.T. 1983. Conservation for an Exhibition of Ancient Chinese Objects: A Case History. AIC Preprints Pg. 18-29.
14. Barton, Gerry and Sabine Weik. 1986. Ultrasonic Cleaning of ethnographic Featherwork in Aqueous Solutions. Studies in Conservation 31(3): 125-132.
15. Cooke, W.D. 1989. A Pilot Study in the Use of Ultrasonic Cleaning in Textile Conservation. The Conservator 13: 41-8.
16. Baird, D.C. 1962. Experimentation: An Introduction to Measurement Theory and Experimental Design Prentice-Hall, Englewood, NJ.
17. Organ was using ultrasound in conjunction with electrochemical reduction of corrosion products on bronze. The ultrasound functioned in speeding the cleaning action of water, flushed out unreacted reagents, as well as providing a finer final treatment. He warned in the report of minute flakes being removed from corroded metal and of the possibility in the process of cleaning corroded objects with ultrasound in acid solution of electrodeposition of fine-grained copper.
18. Firth, J.N.M. 1960. The Cleaning of Fossils by Ultrasonic Waves. Museum J., London, 60(1): 17-18.
19. In pages 436-443 of Design for Scientific Conservation of Antiquities, IIC, Smithsonian Press, Washington, 3968, Organ provides basic directions for the set up of ultrasound devices including magnetostrictive transducers, as well as cautionary notes on overcleaning and draw-backs of cleaning media. He alerts the reader also to the necessity for close examination of the cleaning process.
20. Bettembourg, J.m. 1972. Chemical and ultrasonic Cleaning of Stained Glass, in Book, Compte Rendu du 8e Colloque du Corpus Vitrearum Medii Aevi, York-Cambridge-Canterbury, 25 Sept. - 1 Oct. LRMH. Pg. 47, Champs sur Marne, ICOMOS.
21. Peterson, Karen Stemann and Anne Sommer-Larson. 1983. Cleaning of Ethnographic Feather Garments. Meddelelser om Konservering 3(6): 201-216.
22. Abdulla, Riaz F. 1988. Ultrasound in Organic Synthesis. Aldrichimica Acta 21(2): 31-42.
23. A similar change of phase is described in an article published in 1982, "Interpretation of the Magnified Image of Paint Surfaces and Sample in Terms of Condition and Appearance of the Picture" in IIC Preprints of the Washington Congress on Science and Technology in the Service of Conservation, by Joyce Plesters, Roy Ashok and David Bomford. It tells us that vermillion (HgS) converts under the influence of light from a red form of mercuric sulfide (cinnabar, HgS, hexagonal system) to the black form (metacinnabar, HgS, cubic system), "of identical chemical composition but different crystalline form." Page 170.
24. Grattan D.W. 1989. Personal Communication.
25. Again, in terms of the transfer of heat, ultrasound can be seen to transfer heat by the propagation of waves which transfer energy through a space; by conduction where molecules energized by such waves transfer heat and by convection in liquids and gasses.
26. Robin Chamberlin reporting on the 3-week course on The Consolidation of Ethnographic Objects, held at the Getty Conservation Institute, 11-29 June 1990 tells us that Stefan Michalski presented a method for the consolidation of powdery paint using an ultrasonic humidifier to deliver a 0.5 to 1% gelatin solution to a painted surface. Another example of the proliferation of ultrasonic devices in conservation, WAAC Newsletter, v. 12, #3, 9/1990:19.
27. Although Schumb, W., C. Satterfield and R. Wentworth, in their text, Hydrogen Peroxide, p.70, (1955, Rheinhold, New York), report on several studies which show that hydrogen peroxide formed in water bombarded by ultrasound but only in the presence of a dissolved gas and little hydrogen was produced. Also, depending on the dissolved gas(es) and temperature of the solution, oxygen and hydrogen are formed in various quantities. Ultrasonic irradiation formed hydrogen peroxide in aqueous solutions of various solvents (e.g. carbon tetrachloride), and produced oxidation that could not be attributed to the hydrogen peroxide.
28. Rath, H. and H. Merk. 1953. The Influence of Sonic Vibration on Fibrous Materials. Melliand Textilberichte Int. 34: 6536.
29. Masuda, Katsuhiko, "Kaiga no Kyokubu cleaning ni taisuru suction-table to choonpa hassinki no koka"(Efficiency of a suction-table combined with an ultrasonic transmitter for local cleaning of paintings on silk or paper", Hozon Kagaku, 27, March, 1988:21-4.
30. Katz, Barry J. and Eugene H. Man. 1978. The Effects and Implications of Ultrasonic Cleaning on the Amino Acid Geochemistry of Foraminifera. Biogeochem. Amino Acids Pap. Conf. Pg. 215222.
31. Golbuev, S.V. and Yu D. Semchikov. 1983. Ultrasonic Degradation of Poly-Methyl-Methacrylate in Solution. Izvestia Vysshikh Uchebnykh Zavedenii Khim. I Khim. Tekh. 26(12): 1483.
32. Smith, Merrily A.; Norvell M.M. Jones; Susan L. Page; Dirda Marian Peck. 1984. Pressure-Sensitive Tape and Techniques for its Removal from Paper. JAIC 23(2): 101-113.
33. Appelbaum, Barbara. 1987. Criteria for Treatment: Reversibility. JAIC 26(2): 65-74.
34. Caldararo, Niccolo. 1988. Paper Treatments. AIC Newsletter May: 10-11. References printed in Aug. 1988.
35. Two other studies worth mentioning in this regard which compare numerous modalities are: Geoffrey Michael Lemmer, 1972, The Cleaning and Protective Coating of Ferrous Metals, Bulletin AG-IIC, 12(2): 97-108; and Debra Daly's An Investigation into the Use of Several Substances as Fixatives for Works of Art in Pastel. 9 Master's Thesis, Queen's University, Ontario, 1978.
36. Rose, Ingrid, Yvonne Efremov and Mihai Lupu. 1987. Microscopic Examination of Art on Paper During Treatment in Order to Determine the Effects of Treatment Methods on Fibers and Pigments. Working Group 14, ICOM Committee for Conservation, Getty Conservation Institute, Los Angeles. Pg. 719-726.
37. Pearlstein, E.J.; D. Cabelli; A. King and N. Indictor. 1982. Effects of Eraser Treatment on Paper. JAIC 22(1)1-12. Earlier similar studies were carried out by McCrone Associates, "The Report on Testing Book Cleaning Materials." Report to the Library Technology Project 50; July 1966, and Kerry McInnis, "Two Studies in Paper Conservation Practice." ICCM Bulletin 6; June, 1980.
38. In addition, Grattan, D. (1980, "The oxidative degradation of organic materials and its importance in deterioration of artifacts", JIIC-CG 491: 17-26) has raised questions about the validity of aging tests for Conservation (p.21). New tests need to be designed to consider primary variables of importance to conservators.
39.(a) Suslick, Kenneth and Doktycz, Stephen, "Sounding out new chemistry", New Scientist, 3 February 1990:50-53; also (b) Suslick, Kenneth and Doktycz, Stephen, "Interparticle collisions driven by ultrasound", Science, 2 March 1990, v. 247:1067-1069.
40. Suslick and Doktycz(39a) report temperatures greater than 5,000 degrees Centigrade at local "hot spots" during ultra-sound radiation, the duration of which may be less than one micro-second. Other unexpected situations are created such as where molecules break up under intense temperature and pressure forming reactive fragments which are rapidly cooled, these rates of cooling can reach 1,000 degrees per second.
41. Jets formed by collapsing bubbles can travel at velocities of up to 400 kilometers per hour and collide with the same force as solid objects. Shock waves created by these implosions have been likened to "depth charges" (39a), traveling at or above the speed of sound, containing pressures as great as 3,000 atmospheres.
42. American Institute for Conservation, Paper Conservation Catalog, 1989, 6th ed.," Backing Removal", Chapter 24; and Ellen McCrady, "Steam cleaning book spines", Abbey Newsletter, v. 5, # 6, p. 79, Dec. 1981.
Acknowledgements
I wish to thank Robert M. Organ for reading several drafts of this article and essentially editing the final text. Also, I must thank Lisa Erdos, a protein chemist at Syntex Corporation and D.W. Grattan of the Canadian Conservation Institute for reading the text of the article and offering valuable criticism. Of course, any errors or conclusions are entirely my responsibility.
by Niccolo CaldararoConservation Art Service
Publication History
Received: Fall 1992
This paper was submitted independently by the author, and was not delivered at the Book and Paper specialty group session of the AIC Annual Meeting. It has not received peer-review