The Teas Chart
Foundation for Advancement in Conservation

© 2008
Introduction
Conservators use a variety of solvents to remove adhesives and clean many types of materials. The Teas chart is one of the ways of categorizing solvents. It is mainly empirical, but seems to work and is relatively easy to understand.
Not all conservators agree. For an opposite opinion read: Stavroudis, Chris; Blank, Sharon; "Solvents and Sensibility"; WAAC Newsletter 1989, 11(2), 2–10.
You will need to know basic organic chemistry and functional groups. Organic Chemistry I for Dummies by Arthur Winter is a useful information source.
Contents
This tutorial is divided into the following sections:
Please complete each section in order, as the information builds on that covered in previous sections. You can return to any section later.
Press Ctrl+Shift+F to search.
Learning Objectives
After completing this tutorial, you will be able to:
- Describe the types of intermolecular bonding that are affected by solvents
- Understand the contribution of electronegativity to bond polarity and intermolecular bonding
- Use references that list the Teas fractional parameters to place the solvents on a Teas chart
- Use a Teas chart to aid in selecting appropriate solvents for specific applications
Chemical Bonds
In this section, you will learn that:
- Metallic and covalent bonds are not normally affected by solvents.
- When used to soften adhesives, the solvent is often not used in excess.
- Bonds that involve Van der Waals forces are affected by solvents.
Chemical Bonds: Not Affected by Solvents
 Metallic bonds
Metallic bonds
 Covalent bonds
Covalent bonds
To understand why some solvents work and others don't we need to look at molecular bonding.
First of all let's look at two kinds of bonds that we will not be discussing.
Metallic Bonds: The atoms in metals form lattices of ions surrounded by a sea of delocalized electrons.
Covalent Bonds: The electrons are shared between the nuclei.
Normally these bonds are not affected by solvents.
Chemical Bonds: Solvents
A solvent (and this is usually a liquid) can dissolve another substance (often a solid) to form a solution.
Normally the solvent is the component of a solution that is in excess. This is not often the case in conservation where solvents are used to soften adhesives or remove stains.
Chemical Bonds: Van der Waals Forces
Van der Waals forces (inter-molecular bonding) are the kinds of bonds that are important for solvent action. There are several types of Van der Waals forces:
- London Dispersion Forces
- Debye Forces
- Keesom Interactions
- Hydrogen Bonding
Van der Waals: London Dispersion Forces

London dispersion forces are present in all molecules but may only play a very small part in the overall bonding. The electron cloud around the nuclei of a molecule is in a dynamic state. It fluctuates and moves very quickly (~10-15 sec)
At any one instant there may be more electrons at one end of the molecule than the other. This causes a transient dipole and will also cause an opposite dipole on any molecules in the neighborhood.
Van der Waals: London Dispersion Forces

Despite the fact that these forces are small and transient there are millions of them present. They are the main bonds holding polythene together.
Van der Waals Forces Continued
Debye Forces: These are attractive forces that occur when a non-polar molecule is near another with a permanent polar charge.
Keesom Interactions: Permanent partial charges caused by the unequal sharing of electrons within a molecule.
Hydrogen Bonding: A hydrogen atom attached to a strongly electronegative atom such as oxygen, nitrogen, or fluorine will have a permanent partial positive charge.
Van der Waals Forces: Electronegativity
The concept of electronegativity was first proposed by Linus Pauling in 1932. Electronegativity is relevant to Van der Waals forces because the greater the difference in electronegativity between two atoms, the more polar the bond that will be formed between them. Molecules with permanent dipoles will attract each other more than they would if they had to rely only on dispersion forces.
Chemical Bonds: Periodic Table and Electronegativity
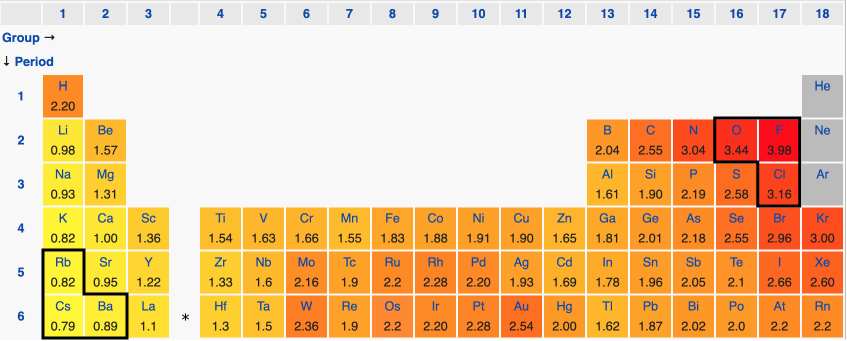
The elements in the top right hand corner of the periodic table—oxygen, fluorine, chlorine—are far more electronegative than those in the bottom left hand corner—rubidium, cesium, barium.
Chemical Bonds: Summary
In this section, you learned that:
- Metallic and covalent bonds are not normally affected by solvents.
- When used to soften adhesives, the solvent is often not used in excess.
- Bonds that involve Van der Waals forces are affected by solvents.
Teas Fractional Parameters
In this section, you will learn that:
- Teas fractional parameters are based on the theory that the intermolecular bonding properties of any material can be divided into three groups: London dispersion forces, polar attractions, and hydrogen bonding.
- The triangular Teas chart allows the fractional parameters for all three forces to be displayed on one graph.
- The chart can be used to predict which solvents will be miscible and to select a suitable solvent to dissolve or soften an adhesive, medium, or coating.
- The properties of solvent mixtures will be the same as the ratios of the fractional parameters of the two solvents.
Teas Fractional Parameters: Three Properties
The development of the Teas fractional parameters has been well documented in Burke, John; "Solubility Parameters: Theory and Application"; AIC Book and Paper Group Annual 1984, 3. They are based on the theory that the intermolecular bonding properties of any material can be divided into three groups:
- London dispersion forces \(f_d\)
- Polar forces \(f_p\)
- Hydrogen bonding \(f_h\)
Having three properties makes it very difficult to display them on a normal graph with two axes.
These are not measured directly, but are estimated as proportions of the total bonding forces.
The Fractional Parameters
The percentage of dispersion forces in the total amount:
\(f_d = \frac{\delta d}{\delta d\ +\ \delta p\ +\ \delta h}\)
The percentage of polar attraction forces in the total amount:
\(f_p = \frac{\delta p}{\delta d\ +\ \delta p\ +\ \delta h}\)
The percentage of hydrogen bonding forces in the total amount:
\(f_h = \frac{\delta h}{\delta d\ +\ \delta p\ +\ \delta h}\)
The sum of these three values always adds up to 100.
Three Graphs
Teas' solution was to display the fractional parameters on three triangular graphs. The Teas chart is three separate graphs on top of each other.

Teas Chart
The values run from 0–100 on each axis.

Teas Chart
Although it is probably easier to follow in color, the Teas chart is usually shown in black and white.

Teas Chart: References
Many of the solvents used in conservation have published fractional solubility parameters.
Horie, Velson; Materials for Conservation; Butterworth-Heinemann: Oxford, 1987 has a table of values at the back.
Barton, A.F.M.; Handbook of Solubility Parameters and Other Cohesive Parameters, 2nd edition; CRC: Boca Raton, Florida, 1991 has more information than you will ever need. However, since it is a relatively expensive book, you might prefer to order it from your library.
Sample Fractional Solubility Parameters
Here are some examples of fractional solubility parameters (taken from Materials in Conservation by C.V. Horie).
| Solvent | \(f_d\) | \(f_p\) | \(f_h\) |
|---|---|---|---|
| Hexane | 100 | 0 | 0 |
| Toluene | 80 | 7 | 13 |
| Ethyl acetate | 51 | 18 | 31 |
| Butan-2-one | 53 | 30 | 17 |
| Ethanol | 36 | 18 | 46 |
| Nitromethane | 40 | 47 | 13 |
| Water | 18 | 28 | 54 |
Notice that, for each solvent,
\(f_d + f_p + f_h = 100\)
Using the Teas Chart

Each solvent has its own unique place on the chart. For example:
| Butan-2-one | |
|---|---|
| \(f_d\) | 53 |
| \(f_p\) | 30 |
| \(f_h\) | 17 |
- First find 53 on the \(f_d\) line
- Then find 30 on the \(f_p\) line and see where the two points meet
- Finally check and see if the value on the \(f_h\) line is correct
Teas Chart: Sample Problem
Place the solvents listed on the chart. When you are finished, go to the next slide to see the answers.| Solvent | \(f_d\) | \(f_p\) | \(f_h\) |
|---|---|---|---|
| Hexane | 100 | 0 | 0 |
| Toluene | 80 | 7 | 13 |
| Ethyl acetate | 51 | 18 | 31 |
| Butan-2-one | 53 | 30 | 17 |
| Ethanol | 36 | 18 | 46 |
| Nitromethane | 40 | 47 | 13 |
| Water | 18 | 28 | 54 |

Teas Chart: Answers to Sample Problem

Effect of Functional Groups

Solvents with similar functional groups will be close together on the chart.
On the whole, solvents increase in polarity, that is they have stronger and more permanent charges, as they move across and up from the bottom right corner.
Like Dissolves Like
In most cases, non-polar solvents (from the bottom right corner of the chart) will mix with polar solvents, so long as one of them is in excess compared to the other.
If you try to mix equal parts of polar and non-polar solvents they will usually separate out.
Solvents from the same area of the chart are generally miscible.
"Like Dissolves Like"
A solvent with similar polar bonding to an adhesive, medium, or coating will usually soften or dissolve it. Solvents, or solvent mixtures, from the same area on the Teas chart can often be used for similar treatments.
Solvent Mixtures
It is easy to locate solvent mixtures on the chart and determine how they will behave relative to other solvents.
(There is one notable exception to this. If you add water to the mixture it is very difficult, if not downright impossible, to place it correctly on the Teas chart.)
The properties of the mixture, such as 1:3 toluene:methanol, will be the same as the ratios of the fractional parameters of the two solvents.
If we know the fractional parameters of the original solvents we can work out the fractional parameters of the mixture and mark it on the Teas chart.
Fractional Solubility Parameters of a Mixture
For a mixture of 1:3 toluene and methanol (0.25 toluene : 0.75 methanol), add together the ratios of the fractional solubility parameters for each solvent.
| \(f_d\) | \(f_p\) | \(f_h\) | |
|---|---|---|---|
| Toluene (total parameters) | 80 | 7 | 13 |
| Toluene (0.25 × total parameters) | 20 | 1.75 | 3.25 |
| Methanol (total parameters) | 30 | 22 | 48 |
| Methanol (0.75 × total parameters) | 22.5 | 16.5 | 36 |
| Add the lines in red to get 1:3 mix toluene:methanol |
42.5 | 18.25 | 39.25 |
Check to see if the final set of values adds up to 100.
Comparison of Two Solvent Mixtures

A 1:3 mixture of toluene and methanol should behave similarly to 2-ethoxyethanol (Cellosolve).
| \(f_d\) | 42 |
| \(f_p\) | 20 |
| \(f_h\) | 38 |
Teas Fractional Parameters: Summary
In this section, you learned that:
- Teas fractional parameters are based on the theory that bonding properties of any material can be divided into three groups: London dispersion forces, polar attractions, and hydrogen bonding.
- The triangular Teas chart allows the fractional parameters for all three forces to be displayed on one graph.
- The chart can be used to predict which solvents will be miscible and to select a suitable solvent to dissolve or soften an adhesive, medium, or coating.
- The properties of solvent mixtures will be the same as the ratios of the fractional parameters of the two solvents.
References
These articles use the Teas chart to categorize solvents. They can be found at JAIC Online.
Phenix, Alan; "The swelling of artists' paints in organic solvents. Part 2, comparative swelling powers of selected organic solvents and solvent mixtures"; JAIC 2002, 41(1). 61–90.
Smith, Merrily A.; Jones, Norvell M. M.; Page, Susan L.; Dirda, Marian Peck; "Pressure-sensitive tape and techniques for its removal from paper"; JAIC 1984, 23(2). 101–113.
Woolbrink, Thomas; "The composition of proprietary paint strippers"; JAIC 1993, 32(1). 43–57.
Credits
Researched and written by Sheila Fairbrass Siegler
Instructional Design by Cyrelle Gerson of Webucate Us
Project Management by Eric Pourchot
Special thanks to members of the Association of North American Graduate Programs in Conservation (ANAGPIC) and the AIC Board of Directors for reviewing these materials.
This project was conceived at a Directors Retreat organized by the Getty Conservation Institute and was developed with grant funding from the Getty Foundation.
Converted to HTML5 by Avery Bazemore, 2021
© 2008 Foundation for Advancement in Conservation