Soaps, Surfactants, and Detergents
Foundation for Advancement in Conservation

© 2008
Introduction
Cleaning is an important part of art conservation. This tutorial reviews essential information about a variety of chemicals used in cleaning artwork.
Before starting this tutorial, refresh your memory on covalent bonding, ionic bonding and hydrogen bonding in a basic chemistry book, such as Organic Chemistry I for Dummies by Arthur Winter.
After reading through this tutorial you might find Science for Conservators: Book 2 Cleaning useful. It gives a good, general background on the nature of dirt and different methods of cleaning.
Contents
This tutorial is divided into the following sections:
Please complete each section in order, as the information builds on that covered in previous sections. You can return to any section later.
Press Ctrl+Shift+F to search.
Learning Objectives
After completing this tutorial, you will be able to:
- Differentiate between soaps and detergents
- Describe in general how soaps and detergents work as surfactants
- Be familiar with some of the advantages and disadvantages of different types of soaps and detergents
Soaps, Surfactants, and Detergents
The title of this tutorial is slightly misleading. It implies three different substances. Soaps and detergents act in a very similar way but are chemically different. They both work as cleaning agents.
However, both soaps and detergents will act as surfactants when in low concentrations. A surfactant (surface acting agent) acts at the interface between different substances to lower the interfacial tension.
A soap and a detergent are both cleaning agents but are defined by their chemistry. Either a soap or a detergent can also be a surfactant in low concentrations.
Soaps
In this section you will learn:
- Three types of soaps
- Most common fatty acids in soaps
- Most common sources of fatty acids in soaps
- Difference between soaps and detergents
Soaps: Three Types
Soap: the sodium or potassium salt of a fatty acid
Metallic soap: the copper, aluminum, lithium, or calcium salt of a fatty acid. Metallic soaps are not water soluble.
Resin soap: alkali salt of a resin
Fatty Acids
Fatty Acid: a long chain hydrocarbon with a carboxylic acid end
The most common fatty acids used in soap making are:
| Palmitic (hexadecanoic acid) |
CH3(CH2)14COOH |
| Stearic (n-octadecanoic acid) |
CH3(CH2)16COOH |
| Oleic (cis-9-octadecanoic acid) |
CH3(CH2)7CH=CH(CH2)7COOH |

Notice that the last fat has a double, or unsaturated, bond. This can act as a reactive center.
Oils in Soaps

The oils that are the starting point for soaps will usually be mixtures of different fatty acids. Olive oil for instance is mainly a mixture of oleic and palmitic acids.
Soaps are made by replacing the acidic hydrogen with either a potassium or sodium cation.
Soft soaps contain potassium salts.
Hard soaps contain sodium salts.
Soaps: Disadvantages
Unfortunately, in hard water soaps will react with the ions of:
- Magnesium
- Calcium
- Iron
They produce complexes that are insoluble in water and precipitate out as soap scum.
One way to overcome this disadvantage is to replace the simple -OH end of the soap with a more complex group that does not form complexes with the ions in hard water. This can be done either at the carboxylic end of the molecule or along the hydrocarbon chain. These are the detergents.
Soaps: Summary
In this section you learned:
- Three types of soaps
- Most common fatty acids in soaps
- Most common sources of fatty acids in soaps
- Difference between soaps and detergents
Detergents
In this section you will learn four broad categories of detergents:
- Anionic detergents
- Sulfates of fatty alcohol or ether
- Sulfonates with the sulfonate group at the end or along the length of the alkyl chain
- Non-ionic detergents
- Cationic detergents
- Amphoteric detergents
Anionic and non-ionic detergents are the ones most often found in conservation treatments.
Anionic Detergents: Sulfate and Sulfonate Groups
Anionic detergents may contain sulfate or sulfonate groups.
| Sulfate group |  |
| Sulfonate group |  |
Anionic Detergents: Sulfates
Fatty alcohol (or ether) sulfates:

Sodium lauryl sulfate
(made from plants)
or
Sodium dodecyl sulfate
(made from petrochemicals)
The —OH end group from the soap has been replaced with the sulfate group to form a detergent.
These detergents produce a rich foam and are used in shampoos, laundry detergents, and carpet shampoos.
General Formula of Detergents: Sulfates
The general formula for these detergents is:

Where:
R = long chain alkyl
A+ = Alkali ion
n is between 1–3
The numerous oxygen atoms mean that the detergent is soluble in water through hydrogen bonding.
Anionic Detergents: Alkyl and Alkane Sulfonates

These detergents were developed as heavy duty detergents with high foaming properties.

Instead of replacing the end group the sulfonate group is attached along the whole length of the alkyl chain. These detergents are biodegradable.
R = alkyl chain
Anionic Detergents: Benzene Sulfonates
In this example the benzyl sulfonate group replaces the carboxylic acid end group. Sulfonate groups can also be attached along the alkyl chain (linear alkyl benzyl sulfonate).
These detergents are biodegradable. They are not however particularly stable in hot water.

Non-Ionic Detergents
These detergents do not have a permanent charge. The water soluble group is usually poly(ethylene oxide).
This type of detergent is used commercially in food and drinks, (where they are used as emulsifiers), pharmaceuticals, and skin care products. They have good cleaning properties at low temperatures. The general formula for these detergents is:

n can be between 10–100 units.
The alkyl chain can be any length.
Environmental Impact of Non-Ionic Detergents
There have been some worries about the environmental impact of non-ionic detergents. Although they are often used at very small concentrations in most pharmaceutical or food products, they are present in large concentrations in laundry detergents. The possible breakdown products in sewerage and waste water have caused some concern.
They may be toxic to some bacteria.
They are suspected of being estrogen mimics in waste water.
The result is that, although the amount of non-ionic detergents used in conservation is extremely small, they are being phased out of commercial use.
Cationic Detergents
These are usually amine complexes and have a positive charge.
Cationic detergents are used as antiseptics in soaps and mouthwash.

Bad Hair Day?

Cationic detergents are added to shampoos and conditioners. They stick to your hair and are difficult to wash out. The slight positive charge makes the individual hairs repel each other.
Amphoteric (Zwitterionic) Detergents
These have permanent positive and negative charges within the same molecule. Amphoteric detergents have good detergent properties over a wide pH range.
Commercially they are considered specialty detergents.
An example of a simple amphoteric detergent:

Detergents: Summary
In this section you learned four broad categories of detergents:
- Anionic detergents
- Sulfates of fatty alcohol or ether
- Sulfonates with the sulfonate group at the end or along the length of the alkyl chain
- Non-ionic detergents
- Cationic detergents
- Amphoteric detergents
Surfactants
Surface Active Agents
In this section you will learn that surfactants:
- Consist of hydrophilic and hydrophobic parts
- Lower interfacial tension
- Work as detergents
- Form micelles and what they do
Surfactants: Hydrophilic and Hydrophobic
We have actually been looking at surfactants throughout this tutorial. All soaps and detergents are classed as surfactants.
All surfactants have:

A "water-hating" (hydrophobic) end.
This is usually a long chain alkyl with no groups or atoms that would hydrogen bond with water.
A "water-loving" (hydrophilic) end.
This could contain permanent charges or atoms that will hydrogen bond with water.
Lower Interfacial Tension
Surfactants act at the interface between different substances:
- The boundary between oil and water
- The boundary between water and air
- The boundary between water and a solid surface such as glass.
This action lowers the energy of the interfacial tension between the two substances.

The hydrophilic end stays in the water.
The hydrophobic end stays in the oil.
How a Surfactant Works as a Detergent
When the surfactant reaches a high enough concentration in the water it will form micelles (critical micelle concentration).
The critical micelle concentration varies between different detergents.

This is a two dimensional diagram of a three dimensional object. The hydrophobic ends are hidden inside the outer ball of hydrophilic ends.
How a Surfactant Works
Once the critical micelle concentration has been reached the surfactant will start to act as a detergent or cleaning agent.
- The surfactant lowers the energy of the interfacial tension.
- Agitation causes the dirt to break into small particles.
- The particles are kept in solution inside the micelles.

Micelle Formation
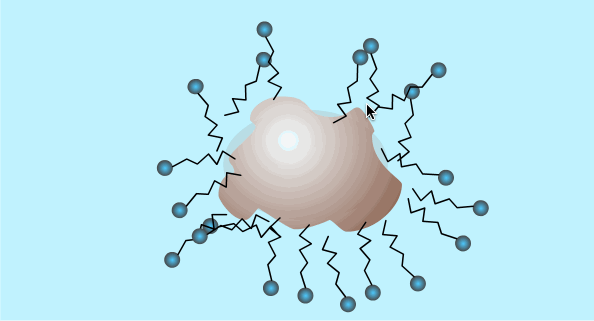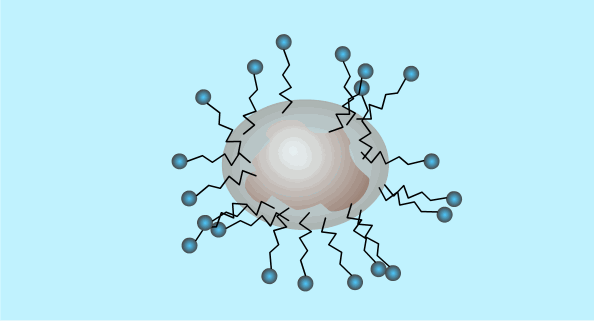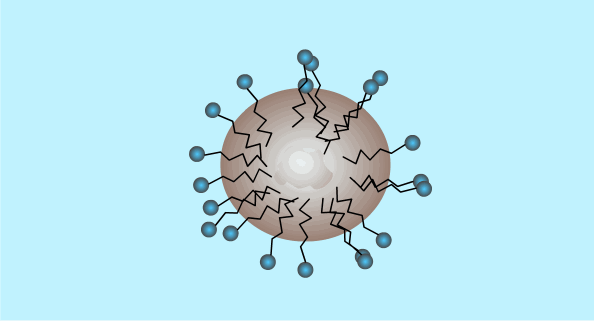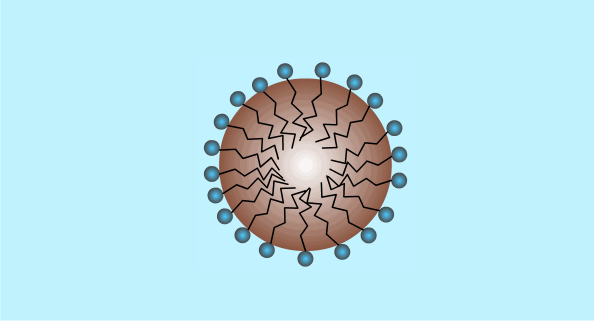
Surfactants: Summary
In this section you learned that surfactants:
- Consist of hydrophilic and hydrophobic parts
- Lower interfacial tension
- Work as detergents
- Form micelles and what they do
References
This article can be found at JAIC Online.
Credits
Researched and written by Sheila Fairbrass Siegler
Instructional Design by Cyrelle Gerson of Webucate Us
Project Management by Eric Pourchot
Special thanks to members of the Association of North American Graduate Programs in Conservation (ANAGPIC) and the AIC Board of Directors for reviewing these materials.
This project was conceived at a Directors Retreat organized by the Getty Conservation Institute and was developed with grant funding from the Getty Foundation.
Converted to HTML5 by Avery Bazemore, 2021
© 2008 Foundation for Advancement in Conservation