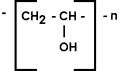
Information and Directions for Production and Use
Chris WoodsPolyvinylalcohol is commonly termed PVA. This should not be confused with polyvinylacetate which should strictly be referred to as PVAc. It is sold by the major chemical companies as a clear granular material in a variety of molecular weights. It is, in effect, a refinement of PVAcetate since the most common manufacturing process is to replace by hydrolysis (or alcoholysis) the acetate groups with hydroxyl groups. This is commonly achieved using the presence of catalytic quantities of alkali such as sodium hydroxide (which, since it acts only as a catalyst, should not in theory remain in the final product). The extent of hydrolysis will determine the amount of residual acetyl groups and this in turn apparently effects the viscosity characteristics. We have been using PVA 22,000 (the number is the molecular weight) which appears to be the lowest weight with the highest degree of hydrolysation (97.5-99.5 mol%). There is a lower weight PVA (15,000) but this has a hydrolysation figure of 87-89%. The viscosity of the heavier weights may render them useless in our context, but I have yet to test any. . .
PVA exists only as a polymer; a monomer has not yet been isolated, so the chemical structure is described thus:
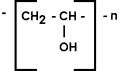
The structure is repeated and 'n' refers to the number of
repetitions in the polymer.
After trying various mixtures, including adding plasticisers, and different cooking methods, we finally returned to the most simple mixture, which can be used for so many of our tasks. For a 20% solution:
200 grams PVA powder
< 1000 millilitres purified water
Place the PVA in a measuring flask and top up with water to 1000 ml total solution. Bring to the boil and simmer for 5 minutes in a microwave oven. If you do not have access to a microwave oven, you must simmer the solution for at least half an hour at as high a temperature as possible without boiling over. PVA needs this intense heating if the granules are to dissolve effectively. If you find there still appears to be granules in the solution after cooking, return it to the heat and continue.
Once the heating is complete, the 20% solution will be a clear, colourless, slightly viscous liquid. On cooling it will be found to be a stiff jelly. It can be used with a brush in its set jelly form or can be 'whizzed' up into a thick liquid by using an electric blender. It can be used in either form for a variety of tasks; we usually use it for making boxes and some display mount systems, where it sticks board to board and is very effective. We also use it to make our own archival paper envelopes for storing documents and find that it is ideal, since it does not wet the paper too much. It has good 'slip' properties for a short while, so boards can be moved about into position. It sticks surfaces together quickly, particularly when under pressure during initial drying. One of my colleagues tells me it takes dyes well and might be used when treating damaged book boards where such toning might be required.
I cannot describe its degree reversibility, having not carried out any serious tests, but brushes inadvertently left for days with the adhesive still on them have been washed clean in water and I am informed that polyvinylalcohol is added to EVA adhesive to improve the latter's reversibility.
PVA powder costs about ?26 pounds sterling for one kilogram. At 20% solutions this equates to 5 litres of adhesive, so I think it is quite cost effective. While the adhesive in solution has no protection from mould and should be kept in a refrigerator, it can be made up from the dry powder when required, so it does not have the short shelf-life of ready-made PVAcetates sold as liquid. It has a neutral pH (usually it reads the same as the water used in making it) and does not contain unstable material as far as I can establish. This is in marked contrast to the PVAcetates which start to loose their acetate groups as acetic acid vapour from the moment they are produced and after six months or so often have a pH of 5 or less.
Name Index no. 7745, The Merck Index, twelfth edition, Merck & Co. Inc. 1996 ISBN 0911910-12-3, Lib. Congress Card 89-60001 Fluka/Sigma catalogue, (9002-89-5) EC No 2091833, product no. 81382 Miall's Dictionary of Chemistry, fifth edition, Longman Group 1981 ISBN 0-582-35152-9
I would like to acknowledge the invaluable assistance of my conservation staff who have cheerfully used whatever slop I have dished up for them and constructively told me how it behaves in use. In particular I am grateful to Trevor Miles for testing different recipes and carrying out most of the initial trials.