Related version of manuscript available as PDF
A formality albeit an important one: This paper was originally published under the title above in The Book and Paper Group Annual, Volume 11 [HTML version] [PDF version] (Washington, DC: The Book and Paper Group of the American Institute for Conservation of Historic and Artistic Works, 1992), pp. 24-33. Accordingly, some copyright formalities need to be mentioned. The entire volume of any B&PG Annual is copyrighted, but publication in the Annual leaves copyright and republication right in the hands of the original author(s). Accordingly, the following note must be appended to any re-distribution of this HTML version of this paper:
© 1992,2002, Hal Erickson
A change-of-address note: As part of an effort to keep the text as close to a verbatim copy original publication as possible, within the formatting limitations of HTML, I have left the address below as it appears in the original publication. However, the current mailing address would be: Preservation and Conservation Studies - Center for the Cultural Record; Graduate School of Library and Information Science; University of Texas at Austin; SZB 564; Austin, TX 78712-1276 and the current e-mail contact would be erickson@physics.utexas.edu
And, finally, a note on pagination: In order to preserve the original pagination, page number corresponding to the original print version have been inserted in the text at the point where each page's text ended, in the following format: « « end of B&PGA 11: p. 23 » ».
Abstract: The results of spectroscopic investigations of reversible and irreversible enzyme binding to paper are reported, as well as the results of surface spectroscopic investigations of the extent to which such residues can be removed by rinsing. As much as 10% of the a-amylase present during a treatment under typical, non-stringent conditions may remain bound to a paper artifact after ethanol denaturation. Less than 30% of such denaturation residues were removed by subsequent washes with water, ethanol, or a water/ethanol 50% (v/v) mix. Alternative methods of inhibiting enzymes without affecting enzyme solubility are proposed, as are alternative methods of removing enzymes. Another common problem with enzyme usage is that overly strong concentrations and elevated temperatures are used to compensate for reduced effectiveness of the enzymes under indifferently chosen treatment conditions; criteria for selection of enzymes and adoption of treatment conditions are suggested. Animal, cereal, fungal and bacterial a-amylases are reviewed and fungal and bacterial a-amylases are recommended for various applications. Formulas and sample calculations for bath immersion, topical, and viscous media applications are presented. Use of acetate buffers rather than phosphate buffers is proposed.
INTRODUCTION:
The proteins capable of catalyzing physiologically important
biochemical processes are known collectively as enzymes. Among
these are a group whose function is catalyzing hydrolytic cleavage
(digestion) of such biological polymers as proteins, starches and
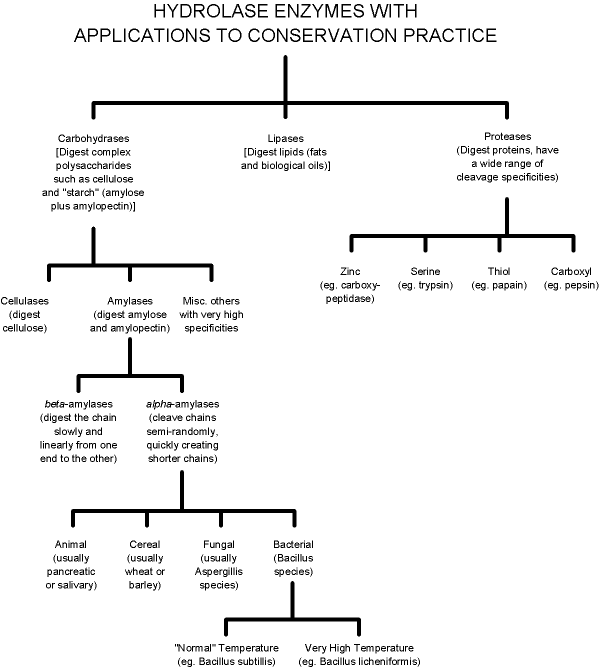
fats. These are known as hydrolases (see Figure
1). The focus of this paper will be the hydrolases whose
function is the digestion of complex polysaccharides (carbohydrates)
such as starch and cellulose. These enzymes, known as
carbohydrases, are highly specific, each catalyzing the hydrolytic
scission of a specific glycosidic bond. Amylases are carbohydrases
that catalyze the digestion of the a-D-1,4 glycosidic bonds
in the amylose and amylopectin that are the primary components of
the purified starches used in making paste.(1) The amylases effect a rapid reduction of
the length of the starch polymer. The resulting fragments are
oligosaccharides that are readily soluble in water and that are too
short to retain significant adhesive capability.
These enzymes have been used in conservation for years, usually
with scant effort expended to maximize their catalytic efficiency.
In order to compensate for the amylases' reduced effectiveness, the
concentrations used have often been higher than was necessary.
Treatment of this sort has often been followed by a step in which
the enzyme is inactivated by exposure to ethanol or hot water; the
intent of this usually ineffective and potentially dangerous step is
to disrupt the enzyme's tertiary shape, thereby terminating its
hydrolytic capacity. Under treatment conditions, especially when
ethanol inactivation is used, some fraction of the enzyme present
may bind to a paper or textile artifact as a residue. It should be
noted that the significance of such residues can be and has been
debated, especially in light of the fact that such residues are
essentially identical to those produced in much heavier quantities
by gelatin-sizing. Nevertheless, since the implications - if any -
of such enzyme residues are presently outside the realm of
consensus, it is prudent to minimize the extent of such binding, at
least to the extent permitted by other conservation and curatorial
considerations.
This paper has two primary goals. The first is to report briefly
the results of spectroscopic investigations of the nature and extent
of protein binding to paper artifacts that occurs under typical
treatment conditions, as well as spectroscopic investigations of the
extent to which such residues can be removed by rinsing. The other
objective is to present guidelines for amylase usage that are drawn
from a review of industrial and patent literature. These protocols
are designed to minimize such protein-artifact binding by maximizing
the digestive effectiveness of the amylase. While this article
focuses on applications to paper artifacts, the results are
sufficiently general that they should also be of use in textile
conservation.
ENZYME-PAPER INTERACTIONS:
A recent conservation treatment involving a-amylase and proteases prompted an
investigation of the extent to which such enzymes would reversibly
and/or irreversibly bind with the lignocellulosic structure of
paper. A multiplexing ultraviolet/visible (UV-vis)
spectrophotometer was used to monitor the adsorption of
ethanol-denatured a-amylase to paper.
This work was performed under conditions encountered within
protocols still commonly used by the conservation community.
Analysis revealed that 10% or more of the denatured a-amylase present may bind to the paper object
being treated when denaturation of the enzyme occurs in the presence
of the cellulosic object. Further investigation using a UV-vis
spectrophotometer with surface absorption accessory revealed that
rinsing protocols practiced in the conservation community typically
remove 30% or less of the enzyme that becomes bound to the paper
during these unnecessary denaturation steps. Details of these
results may be found in the Experimental
section of this paper.«« end of
B&PGA 11: p. 24 »»
USAGE CONSIDERATIONS:
The bench conservator's work with enzymes is always constrained
by the professional consensus that treatment must never irreversibly
change an artifact. Two important limitations on enzyme use are
implicit in this ethic. The first is that enzyme residues such as
those described above should be minimized or eliminated - at least
until their consequences are fully understood. This is accomplished
through thoughtful attention to selection of the enzyme and to its
usage requirements. The other limitation is that treatment
conditions when using enzymes must be governed primarily by concern
for the integrity of the artifact, and must not be determined by
usage optima of the enzymes. Given these constraints, many
conservators are understandably hesitant to use these powerful
biomolecular tools. While a healthy dose of such caution is
desirable, it is also important that the conservator also be aware
that judicious selection of a particular a-amylase type and purity, when made with
commensurate anticipation of reaction conditions, can yield safe and
effective treatment for the majority of objects. The discussion
that follows is intended to prepare the conservator to match the
artifact's frail ties and susceptibilities to a particular a-amylase's reaction optima.
The conservation community has been repeatedly cautioned by
Burgess and others not to use pH, temperature and ion concentration
information from the assay parameters supplied with the assay
results, usually on the container's label. These are standardized
conditions that are used to assay any a-amylase, regardless of its source and its
reaction optima. The intent of these standardized conditions is
merely to permit the comparison of enzymes from various suppliers.
Users should exercise similar caution about the use of reaction
optima data from the biochemistry and molecular biology literature,
if it is to be used at all, be cause that data is almost universally
generated in studies in which both the enzyme and the digestible
substrate are dissolved in aqueous solution with careful attention
to ion balance and pH. The applications confronting the bench
conservator, by comparison, are invariably bi-phasic. They take
place at the interface between a solid substrate - typically a
cross-linked adhesive - and a fully or partially solvated enzyme.
Furthermore, the requirements of a particular conservation
application seldom conveniently match the digestion optima of the
enzyme sitting in the freezer down the hall.
Fortuitously, these same problems of reaction-phase heterogeneity
and difficult reaction conditions also confront the brewing, corn
syrup, cheese-making, candy, baking, dairy, meat, seafood
processing, vegetable processing,«« end of
B&PGA 11: p. 25 »»
starch, paper and textile industries. Dozens of volumes of data are
available,(2,3,4,5,6,7,8,9,10,11) as are hundreds of
technical papers and hundreds of patent applications. The
half-billion dollar- per-year enzyme industry generates dozens of
patent applications annually, each of which is supported by masses
of such data. Many of these patent applications involve amylases
and proteases.
I believe that the following summary of that body of industrial
data largely complements and confirms forth coming experimental
results generated independently by investigators at the Canadian
Conservation Institute. The industrial patent literature is rich in
experimental data describing behavior of enzymes under real-world
treatment conditions - the same non-ideal conditions that perplex
the conservator. Among the conditions to which the practicing
conservator must pay close attention are the pH, the calcium and
sodium concentrations, the temperature, and the commercially
available purity of the enzymes. Each of these factors varies
widely and is dependent on the species from which the amylase was
extracted. Conveniently, the industrial labs have already completed
most of the investigations of these factors.
 |
| Figure 1: Partial taxonomy of hydrolases illustrating context of various a-amylases. |
outlines some of the many available variants of amylases. One
of the most important considerations is the choice of a-amylases over b-amylases. b-Amylases are mechanistically exo - that is,
they methodically digest the amylose and amylopectin into di- and
tri-saccharides, working linearly from one end of the polymeric
chain to the other. They suffer from the drawback that they are
stymied when they reach a a-D-1,6 branching linkage in amylopectin, which
occurs on average about once in every 25 linear glucose units, the
remainder of which are joined by the more typical a-D-1,4 linkages. a-Amylases, which conversely are
mechanistically endo, attack the starch polymeric structure
semi-randomly, quickly reducing it to a series of readily-soluble
short oligosaccharides. The closely related glucoamylases and
pullulanases will be encountered in the amylase literature by any
enquiring reader, but these are not well suited to conservation
usage. Glucoamylases hydrolyze both a-D-1,4 and a-D-1,6 linkages, but
are mechanistically exo, making them too inefficient and slow for
conservation treatments. Pullulanases, on the other hand, are
debranching enzymes that only digest the a-D-1,6 linkages that
are responsible for amylopectin's branched structure; they therefore
could be used as a complement to b-amylases, but b-amylase's slow exo digestion eliminates even
the complementary pair from consideration as useful biomolecular
tools.
a-Amylases are extracted from a number
of biological sources. These sources include animal (usually
pancreatic or salivary), cereal (usually wheat or barley),(12) fungal (usually derived from large-scale
fermentations of Aspergillus species), and bacterial (derived
from similar fermentations of Bacillus species).(13) Even within a given species, amylase
optima may vary depending on the organ from which or fermentation
conditions under which the amylase was extracted. Selection is
further complicated by the fact that industrial suppliers of enzymes
have cultivated mutant strains of many popular amylase-producing
species, each of which in turn has different digestion condition
optima.
It will be seen that economics and availability of high purities
will largely dictate the use of fungal and bacterial a-amylases. A detailed discussion is found in
the Specific Usage
Recommendations section.
PRE-TESTING FOR ARTIFACT SAFETY:
In at least one conservation treatment whose results were
examined in preparing for this paper, use of an enzyme had a
substantial negative impact on the object that was treated. In that
particular case, an immersion bath of protease resulted in a sudden
floating of a number of mold-damaged fragments that were apparently
being tenuously held to the bulk of the paper by the adhesive action
of the gelatin size. While this is not a problem likely to occur in
amylase treatments of Western paper objects, it points up the need
for pre-testing, even with a biomolecular tool whose specificity is
as high as that of amylase. It is especially imperative that
workers involved with Islamic paper artifacts act with extreme
caution, since these are known to be traditionally sized, burnished
and even dyed using starch.(14)
Some modern Western papers are starch coated or starch loaded.
Preliminary investigations performed at the Bodleian Library showed
no visible effects of amylase treatment on starch loaded or coated
papers, or on the printing on such papers. The investigators did
report some apparent but unquantified weakening of the
paper.(15) Stringent pretesting then
is definitely merited when such Western papers are encountered.
There is good reason to believe that amylase activity would be
statistically more likely to attack starch incorporated in the paper
than to attack a starch-adhesive residue on the paper, based purely
on the relative number of sites for digestive attack; amylase
treatment is then probably contraindicated in both these cases
because of the low probability of effective treatment and the
significant possibility of ill effects.
PRETESTING FOR ENZYME ACTIVITY:
In an ideal world, conservators would be sufficiently comfortable
with standard molecular biology techniques that they could perform
their own assays of the hydrolytic activity of such enzymes as
amylase and protease. Indeed, this has been recommended.
Unsurprisingly, however, not one of the bench conservators
interviewed in preparation for this paper was comfortable with the
prospect of performing such an assay.
Given the excellent reliability of the enzyme market at present,
particularly in light of the high purities and long shelf lives
available, a few reasonable guidelines are probably sufficient to
permit conservators to assume the acceptability of a given enzyme.
The three guidelines are the following. Always purchase high purity
enzymes, since«« end of
B&PGA 11: p. 26 »»
crude preparations contain unnecessary contaminants, and may even
contain such rogue enzymes as cellulase. Always maintain high
standards of chemical practice when micropipetting from the bulk
container or when transferring lyophilized solids to the scale, in
order to minimize contamination of either the bulk product or the
prepared solution; cross-contamination with protease must be avoided
with particular diligence. Finally, one should always store enzymes
as recommended by the supplier or as described in a later
section.
If a conservator desires a crude but effective test for activity,
then s/he may wish to adopt some form of the following test.(16) Select two sheets of a strong uncoated
paper whose furnish is neither extremely smooth nor extremely rough.
Working quickly, brush out a fairly heavy coat of starch paste onto
each, using paste that has been thinned to about half of its normal
viscosity. Place the paste sides together and allow to dry
completely (one to two days) under pressure. Place the dried
composite sheet into a humid oven at 90-98°C (194-208°F) for
3-7 days until the adhesive is sufficiently cross-linked to be
considered intractable.(17) Cut the
sheet into small strips, each pair of which will permit the checking
of an amylase preparation once. Since this assay should only be
necessary on those rare occasions when long-stored enzymes are
removed from cold storage, a single preparation of such a set of
strips should provide enough material to supply a number of labs for
years.
The assay is performed by simply moistening one strip with an
appropriately prepared enzyme solution and moistening the other with
a control solution differing from the enzyme solution only in the
absence of a-amylase. The pasted halves
of the strip moistened with the enzyme solution should separate
significantly more quickly than those of the strip moistened with
the control solution.
METHODS OF APPLICATION:
While the spectroscopic studies detailed in the Experimental section are focused on bath
immersion, it should be noted that the implications generalize well
to all other common methods of enzyme application. These methods
include, but are not limited to, bath immersion (aqueous as well as
partially or completely nonaqueous), topical or spot application
with swabs or blotter paper, and viscous media (gel or poultice)
application.
In bath immersions, a relatively large volume of dilute enzyme
solution is used. The advantages of this method are that the
enzymes are freely mobile, permitting more facile digestion of the
starch substrate, and that the resulting oligosaccharide fragments
dissolve away from the site of the digestion. The disadvantage, of
course, is the incompatibility of many media with water. This
incompatibility has been overcome in some treatments through the use
of nonaqueous or partially aqueous solutions.(18) The use of carbohydrases in nonaqueous
media require overcoming some particularly difficult biophysical
constraints.(19)
In general, aqueous immersion bath treatments per formed with
adequate attention to ionic concentrations and pH should work with
acceptable speed at concentrations of 1-5 units activity per
milliliter(20) (see sample
calculations, later section), but a number of other factors should
be weighed in choosing the enzyme concentration to be used in a
particular treatment. The dynamic that should be foremost in the
conservators mind is the tradeoff between, on the one hand, the
lengthened digestive time required at sub-optimal pH and temperature
which permits more time for such undesirable effects as offsetting,
and on the other, the potential damage to the object that may result
from attempting to achieve shortened digestion times with elevated
temperature and pH adjustment.
A good rule of thumb for a-amylase
concentrations is viscous media and in solutions intended for
topical application appears to be a minimum tenfold increase in
enzyme concentration over what would be used for aqueous immersion.
It is imperative to remember that a dry immobilized enzyme cannot
perform any useful function. When performing such viscous media or
topical applications, sufficient moisture must be maintained to
yield mobility for the enzyme. Generally speaking, this will be the
point at which the paper fibers have just become fully swollen but
do not contain bulk water. This condition can be achieved in
viscous media applications by using a loose gel or by slight
prewetting; humidity is then maintained by the gel or poultice.
Such a fiber-swollen condition can be maintained for topical
applications by damp blotters, with or without a protective barrier
of Gore-Tex® expanded poly(tetrafluoroethylene) on polyester
backing.
The consensus among book-and-paper conservators appears to be
that topical application is most common, followed by aqueous
immersion. Other methods are rarely if ever used.
TEMPERATURE:
The conservation literature frequently mentions the need to let
enzymes work at elevated temperatures, as well as of the enzymes'
tendency to denature at about 40°C. In fact, the amylases and
proteases are quite robust thermally, with most reaching their
thermal optima in the range of 50- 105°C and losing significant
catalytic activity in the range of 65-110°C. These figures make
two critically important points immediately clear. First, attempts
at denaturation with hot water would be ineffective at the
conventionally recommended temperatures, and would be dangerous to
the artifact at temperatures where effective denaturation is
achieved. Second, temperatures sufficiently elevated to maximize
the enzymes' catalytic activity are likely to be dangerous to the
object.
None of this should be taken to belittle the intended result of
working at elevated temperatures; indeed, digestion rates can be
increased hundred-fold in this way. What the bench conservator
should consider, however, is that similar increases can be achieved
through careful selection of enzymes and through careful attention
to pH and ion«« end of
B&PGA 11: p. 27 »»
balance, while eliminating the logistical difficulties and artifact
jeopardy that result from attempts to work at these
temperatures.
Also, those conservators who perform topical application of warm
enzyme solutions by dipping swabs or blotter paper in a beaker of
enzyme solution that is kept at the benchtop on an electric hotplate
are accomplishing little if any benefit for their extra trouble.
The solution will have largely cooled by the time it is transferred
to the object, and the digestion temperature will be determined by
the temperature of the object. In fact, such a procedure may have a
negative impact since the enzyme will suffer accelerated
deterioration while sitting for hours on the benchtop.
pH CONTROL AND BUFFER SOLUTIONS:
While it is hoped that the guidelines from the Specific Usage Recommendations section
will be adequate to allow the conservator to match an appropriate
enzyme to the treated object's native pH, there may be times when
the pH of the enzyme solution or poultice must be controlled in
order to maintain the activity of the enzyme. Phosphate buffers
have traditionally been used in conservation practice to control
enzyme treatment pH. Phosphates, however, have the unfortunate side
effect of causing precipitation of calcium ions that are needed for
full activity of the fungal and bacterial amylases. Since these
microbiological amylases are economically and chemically best-suited
to conservation treatment, this calcium phosphate precipitation
presents a significant problem.
A good solution would be to use acetate buffers instead of the
phosphate buffers. Data from Wakim, et al., can be used to
demonstrate that no significant change in activity occurs when
buffer systems are changed, at least for porcine pancreatic a-amylase,(21)
which is not so highly calcium dependent.
The acetate buffers are simple to prepare. 0.1 ml of
concentrated acetic acid ("glacial," 99.4-99.8% CH3COOH,
density 1.05g/ml) combined with 1.44 g sodium acetate
(NaC2H3O2) or 2.38 g sodium acetate
trihydrate
(NaC2H3O2·3H2O)
in a liter of solution will yield a simple buffer with a pH of about
5.75. This simple buffer displays only limited resistance to pH
change by strong bases, but it does exhibit excellent resistance to
pH changes by acids, which is a far more prevalent complication in
conservation applications anyway. pH of the buffer can be further
increased to a value as high as ph=8.5 by further decreasing the
volume of glacial acetic acid added to the buffer, although at the
cost of still more limited capacity to respond to an object having a
native pH greater than 8.5. The prime advantage of acetate buffers
is that they do not interfere with the solubility of any metallic
ions, while also having no known conservation contraindications. Of
particular comfort is the fact that acetates are so soluble that
they rinse easily from the paper.
A note in passing about Trizma® buffers is in order. Such
buffers have been recommended to avoid the phosphate precipitation
problem described above, while providing full buffering against both
acids and bases. Unpublished results indicate that Trizma®
buffers may decrease fold endurance of paper. Until this issue is
resolved, acetate buffers appear to be the preferable
alternative.
Most importantly, since the majority of treatments are apparently
topical rather than immersion, the conservator should keep in mind
that buffering of an enzyme solution that is to be used for topical
application is largely a futile endeavor, since the pH of the
digestive process will be determined by the native pH of the
object.
ION BALANCE:
a-Amylases are considered, broadly
speaking, metallo enzymes, i.e. enzymes that require the
presence of a metal cation "cofactor" in order to express their full
activity. In a-amylases, the metal is
calcium. The number of calcium atoms required per enzyme have been
reviewed in many places over the last 3 decades. The many apparent
contradictions in this body of data are the result of an evolving
awareness of the tendency of many enzymes, amylases among them, to
form multi-enzyme quaternary complexes that may on first
investigation appear to be larger enzymes having higher activity and
higher calcium counts. Such issues may now be resolved
unambiguously using x-ray diffraction.
Some a-amylases, notably those derived
from mammalian sources, may also require the presence of other
enzymes such as sodium and calcium. The requirements of various
a-amylases are discussed in more detail
in the Specific Usage Recommendations
section.
The presence of appropriate ions can easily increase the activity
of the enzyme ten-fold or more, allowing the conservator to either
lower the enzyme's concentration to minimize residual binding or to
shorten the working time and thereby lower the likelihood of such
aqueous treatment artifacts as offsetting of media or tidelines.
The conservator, however, should not begin to dread the preparation
of millimolar solutions of various ions in order to tweak the
performance of a particular enzyme. The fungal and bacterial
enzymes are quite robust; the requisite Ca+2 ion in
Asp. species, for instance, appears to be so tightly bound
that it will not be lost even if dissolved in distilled water.
Furthermore, Toda and Narita have established that magnesium ions
will restore the activity of Asp. oryzae a-amylases that have had their calcium removed
by incubation with the calcium-chelator ethylenediaminetetraacetic
acid (EDTA).(22)
CONCENTRATION CALCULATIONS:
The most commonly purchased form of enzymes is a water-soluble
lyophilized powder. The number of grams of enzyme that will be
weighed out, ms, when preparing
V milliliters of an enzyme solution having a
concentration of Cv units activity per ml
solution is given by:«« end of
B&PGA 11: p. 28 »»
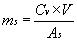 (EQN.1)
(EQN.1)
where As is the activity of the solid
expressed as "units of activity per mg of solid" (as opposed to
"units of activity per mg of protein"). If, for instance, one
needed to make 100 ml of an enzyme solution having an activity
concentration of 5 units/ml from a powder labeled "1100 units/mg
solid," then one would weigh out:
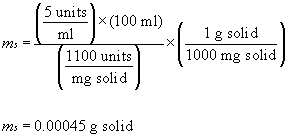 (EQN.2)
(EQN.2)
It will be obvious that the measurement of such small masses will
require an analytical scale having a minimum sensitivity of 0.0001 g
and ideally 0.00001 g. Many labs, having access only to scale less
sensitive than this, may need to adopt the expedient of making
larger quantities of solution and storing the excess, or of making a
concentrated stock solution that can be stored in frozen
aliquots.
Some enzymes, however, are readily available only in a
concentrated liquid form. One purchases a large amount of highly
purified enzyme, say 500,000 units or more, in a volume of 30-500 ml
of aqueous buffer solution. This actually makes the preparation of
solutions easier, as long as one has access to a micropipette
capable of measuring down to at least ten microliters (10 µl).
These micropipettes are available affordably from a number of
sources and should be purchased with a supply of tips that are
ready- packed in their dispenser boxes.(23) The calculation of the number of µl
of concentrate, Vµ, that should be
dispensed when preparing V milliliters of an enzyme
solution having a concentration of Cv units
activity per ml solution is given by:
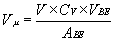 (EQN.3)
(EQN.3)
where ABE is the number of units of
activity of bulk enzyme that were purchased, and where
VBE is the approximate volume of the bulk
enzyme concentrate that was purchased. If one had purchased, let us
say, 500,000 units of B. licheniformis which arrived dissolved in
approximately 35 ml of bulk solution,(24) and wished to prepare 10 ml of a treatment
bath having a concentration of 15 units/ml, then one would measure
out:
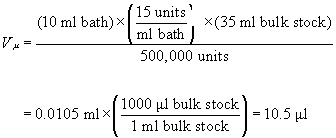 (EQN.4)
(EQN.4)
STORAGE OF ENZYMES:
Amylases and proteases are notoriously robust enzymes. The
conventional wisdom is that if one smeared saliva into a dirty table
and left it to dry fully exposed for weeks, one could still find
significant retention of catalytic activity in an extract of
scrapings from the table. That said, some qualifiers are in order.
Enzymes are traditionally stored at temperatures just above or below
the freezing point of water in order to preserve their activity.
Users must keep clearly in mind that freeze-thaw cycles are one of
the worst culprits in accelerated loss of enzyme function.
Concentrated bulk stock solutions are typically stored refrigerated
at 0-5°C in order to avoid these cycles. Premeasured aliquots
of concentrated stock solutions, or even premixed ready-to-use
solutions, may be stored frozen for six or even twelve months, but
only in a freezer that does not have an automatic defrost cycle.
The only freeze- thaw to which these should be exposed is the
initial freeze and final thaw. Ready-to-use solutions may be stored
refrigerated at 0-5°C for weeks, but users should bear in mind
that an additional factor then enters play. Not only are
traditional modes of inactivation slowly taking their toll, but
precipitation of the protein on the container wall will also be
contributing more rapidly to loss of solution activity. For this
reason, Sigma Chemicals recommends that only solutions with
concentrations greater than 0.5 mg protein/ml solution be stored
refrigerated.(25) Since these
concentrations are significantly higher than those typically used in
conservation practice, this method of storage is recommended only
for premeasured aliquots of concentrated stock solutions.(26) Bulk enzymes received as lyophilized
solids should be stored frozen in a desiccator, or at least inside
multiple plastic bags with a desiccant. Properly stored bulk
amylases, whether solid or concentrated liquid, should lose activity
only at the rate of 1-2% per year.
In summary, then, amylase solids and concentrated liquid
solutions are sufficiently robust to permit long term storage in the
freezer and refrigerator respectively. The two storage problems
that may arise are in the freezing of liquids in an auto-defrost
freezer, and the refrigeration of dilute (working strength)
solutions that will lose strength as enzyme precipitates on the
glass walls of the container.
DEACTIVATION OF ENZYMES:
An enzyme's activity can be disrupted through any chemical,
thermal or physical method that alters the tertiary configuration of
the protein. The three broad classes of inactivation are
inhibition, denaturation and destruction. Inhibition is usually
accomplished by a change in pH or ion balance, or by the
introduction of enzyme-specific inhibition proteins; it results in a
reversible or irreversible blockage of access to and/or shape-change
of the enzyme's active site. Denaturation - a partial or complete
unraveling of the protein's tertiary structure - is usually
accomplished through extreme pH or heat, simple drying, solvents
(such as ethanol or acetone), or surfactants (e.g. sodium
dodecyl«« end of
B&PGA 11: p. 29 »»
sulfate, SDS); the denaturation may be partially or fully reversible
if the denaturant is removed or neutralized and if the denaturation
is not too far progressed. Irreversible destruction involves
chemically changing the nature of the protein; in conservation this
would typically be achieved by permitting the air to oxidize a dried
protein residue or by cleavage with a protease.
The conservation literature has conventionally pre scribed the
denaturation of enzymes with heated water or ethanol. These steps
are unnecessary for several reasons. Foremost among these is the
potential damage to the object that may result from the use of such
harsh denaturation conditions, a consideration that appears in the
literature at least as early as 1977.(27) Supporting this contention is the fact
that the water soluble enzymes will largely wash away if they are
never permitted to dry or denature on the paper, and that any
residues that do dry (as opposed to denature) on the paper will wash
away after brief pre-hydration. Further supporting this contention
is the fact that there exist no known negative effects of a dried
enzyme remaining on the paper after, say, a topical spot application
to a water-sensitive object, other than the difficulty of getting a
starch paste to adhere to that spot in the near future.
SPECIFIC USAGE
RECOMMENDATIONS:
When purchasing enzymes, avoid those described as crude
preparations, since these will be contaminated with other proteins
that bind to the artifact without conveying any additional digestive
activity to the treatment solution. Indeed, crude preparations of
this type may even be contaminated with cellulase that will inflict
significant structural damage on the paper by hydrolytic digestion
of the paper's cellulosic fibers. Purchase only high purity
enzymes, usually described in catalogs as crystalline or
lyophilized.
Burgess has recommended that enzyme concentrations for immersion
treatment of paper objects be on the order of 1-5 units of amylase
activity per milliliter of solution. Her rationale is that these
concentrations are adequate to digest most occurrences of
cross-linked starch adhesives encountered in conservation practice
if careful attention is paid to digestion conditions such as pH, ion
balance, and temperature.(28)
Remember, however, that these recommendations are for optimized
conditions. Little if any detectable residue is likely to be
deposited on the object even at concentrations in the range of
50-250 units activity per ml, as long as in situ denaturation
is avoided. If an adhesive is intractable, do not hesitate to
increase the a-amylase concentration
significantly.
The paragraphs that follow address the issues pertinent to
reaction conditions for the a-amylases of
fungal, bacterial, cereal and mammalian origin respectively.
The most readily available fungal a-amylases are those derived from
Aspergillus oryzae. These are available commercially in
reasonably high purity and display the calcium inactivation behavior
described below, making it a particularly good choice for risky
treatments. The amylase derived from native A. oryzae
displays acceptable activity between pH 5 and 7 with optimum
activity reportedly between 4.8-5.8. Temperature stability extends
to 50-55°C. It is important to note that fungal amylases
require calcium ions for full enzymatic activity; this must not,
however, be construed as a requirement for the addition of calcium
ions to the treatment bath. The calcium ion needed to maintain the
hydrolytic activity of fungal a-amylases
is tightly bound in the active site of the enzyme protein,(29) and the very small concentration of
calcium leached from the artifact should be more than sufficient to
maintain the enzyme's full activity. Fortification of the treatment
bath with calcium ions is likely to inactivate the enzyme as
described in a later section. The a-amylase extracted from cultures of Asp.
niger has similar properties, but is not readily available in
the purities required for conservation practice.
Bacterial amylases have been extensively researched and are
prolifically described in the patent literature. Unfortunately,
only two Bacillus enzymes are readily available to the
conservation community in acceptable purity. Both B.
subtillis and B. licheniformis require sodium ion and
calcium ion in order to preserve the enzyme's full activity. That of
B. licheniformis, however, requires only 5 ppm
Ca+2, while that of B. subtillis requires 150 ppm
Ca+2. The heat stability of B. subtillis amylase
extends as high as 80-85°C and that of B. licheniformis
extends to 110°C; both species' amylases nevertheless show
comparable activities at room temperature.
Cereal a-amylases, such as those
derived from barley malt or wheat are available only in crude form,
which makes these enzymes unsuitable for most conservators. For
conservators with access to preparative-scale protein
chromatographic apparatus, these crude preparations do, however,
offer an economical source of purifiable amylase. The user should
be aware that cereal amylases display a pronounced shift in pH
optima with change in temperature. For instance, the pH range of
optimum activity of amylase derived from barley malt will shift from
pH 4.7-5.4 at 50- 55°C to pH 5.6-5.8 at 70-75°C. The
conventional rule of thumb is that cereal a-amylases display their highest activity
between pH 5-6.(30)
Mammalian a-amylases, such as those
extracted from beef or porcine pancreas or salivary gland, or indeed
those from human saliva, are available in very high purity, but are
prohibitively expensive, without offering additional advantages.
Their use is further complicated by the need to supply chloride ions
in order to achieve full enzymatic activity. While their optimal
activity is traditionally said to be around pH 7, this activity can
decline sharply and show a substantial loss of breadth of pH
activity when chloride ion is absent. Porcine pancreatic a-amylase, for instance, has been shown to
decrease activity by an order of magnitude when
Cl--enrichment of 0.025 M is
not«« end of
B&PGA 11: p. 30 »»
provided, while simultaneously decreasing its range of acceptable
activity from pH 5-10 to pH 5-6.(31)
For conservation purposes, then, the issues of function, purity
and economics alone are sufficient to limit the choice of available
carbohydrases to fungal and bacterial a-amylases. The currently available options
are a-amylases from B. subtillis,
B. licheniformis, and Asp. oryzae, of which the most
flexible, easy-to-dispense, and rapid working is B.
licheniformis. Conservators looking for a single multi-purpose
enzyme will do well to consider purchasing a micropipette and
beginning to use B. licheniformis a-amylase.
URGENT ARREST OF ABERRANT TREATMENT:
Recent research(32) indicates that
the activity of a-amylases derived from
such fungal species as Aspergillus oryzae can be effectively
terminated using calcium ions - a reversible inhibitor. These
investigators found that calcium concentrations of 20 millimolar
(800 ppm Ca+2) were sufficient to reduce the enzymatic
activity of fungal amylases by 99%. This offers the conservator who
is attempting a high-risk immersion treatment the opportunity to
arrest the treatment's progress in the event of undesirable effects.
The conservator can prepare a 2 molar Ca+2 stock
solution, premeasured in a volume equal to one-hundredth of the
volume of the treatment bath; a 2 M Ca+2 solution may be
prepared by dissolving 2.22 g calcium chloride, CaCl2, or
2.94 g calcium chloride monohydrate,
CaCl2�·�H2O, in 10�ml of
distilled water. This solution is extremely stable and can be kept
for future use in a tightly sealed container. A 10�ml aliquot
should be prepared and ready for every 1 L volume of the bath. The
conservator is then prepared to quickly inactivate the fungal a-amylase by pouring the calcium stock solution
into the treatment bath with gentle agitation. The artifact may now
be removed carefully from the bath, taking time to avoid physical
damage, with the knowledge that further amylase-inflicted damage
will not occur while the time is being taken to exercise this
caution.
ENVIRONMENTAL HEALTH AND SAFETY ISSUES:
Enzymes present very few dangers to the user other than as an
inhaled irritant and potential inhaled allergen. Skin absorption or
ingestion are unlikely to occur and present only low hazard if they
should occur. Keep clearly in mind that amylase and protease are
two of the primary active ingredients of saliva.
Standard industrial hygiene precautions for handling low hazard
friable, air-suspendable, powdered solids should be taken when
transferring and weighing solid enzyme preparations. Standard
precautions would include the wearing of an appropriately fitted
respirator by any user with a known or suspected allergy to
amylases. The suppliers' Material Safety Data Sheets (MSDS) may be
consulted for further details, but the user should keep in mind that
MSDS's for proteins such as enzymes tend to be filled with generic
boilerplate text. The hazards of individual proteins cannot be
adequately investigated for the thou sands of commercially available
proteins, each of which is sold only in very small quantities. This
results in MSDS's that tend to be written for the worst possible
case, whether or not the available evidence supports the recommended
levels of industrial hygiene.
Spills of either the solutions or solids present no great hazards
other than the minor health considerations described above.
Solutions of amylase are roughly as dangerous as saliva and may be
disposed of in approximately the same manner. Large quantities of
unused solids should be disposed of through an appropriate hazardous
materials disposal program, while small amounts of spilled or unused
amylase can be safely wrapped in damp paper towels and thrown away.
These practical guidelines may be superseded by legal requirements
in communities, states or countries with broad and stringent
regulations covering disposal of "chemicals" in the sanitary sewer
or municipal landfills.
A Hewlett-Packard HP8450A multiplexing UV-vis spectrophotometer
was used to monitor the adsorption of ethanol-denatured amylase to
paper. This work was performed using a variety of standard and
historical papers under conditions encountered within protocols
still in common use by the conservation community. An 0.1% (w/v)
solution of amylase was prepared. The amylase used was Sigma
Catalog No. A 6380, Type IIA, which was received as a four-fold
recrystallized solid having 1400 units of activity per mg of
solid;(33) the enzyme had been
supplied to Sigma as being of B. subtillis origin, but Sigma
included a disclaimer in the catalog suggesting that their
investigation indicated that the source would more properly be
described as B. amyloliquifaciens.
The results indicate that a significant percentage of the
denatured amylase will bind to a paper sample that is present at the
time of denaturation. This effect is absent when paper is added to
a solution of native enzyme and is only minor when the paper is
added after denaturation, indicating that the enzyme-artifact
binding primarily takes place immediately after denaturation, before
partial renaturation can occur. More detailed data on the extent of
the enzyme-artifact binding to a variety of historic and modern
papers was sought, but the extent of UV-absorbing leachate from most
papers was of such magnitude and variability as to overwhelm and
obscure the relatively small spectral changes being monitored.
Whatman #3 Chromatography (W3C) paper (Cat. CP3MM, basis weight 185
g/m2, thickness 0.33 mm), however, was shown to have only
a negligible UV-absorbing leachate. This permitted observation of a
quantitatively reproducible effect. When 2 ml of standard 0.1%
(w/v) amylase aqueous solution(34) is
denatured with 1 ml of ethanol in the presence of 6.25
cm2 of W3C paper, 11.6 ± 0.3 % of the 2 mg of
amylase present were bound to the paper. These results indicate
that in situ denaturation is undesirable under almost all
circumstances.«« end of
B&PGA 11: p. 31 »»
When deactivation of the enzyme can be avoided - which should be in
virtually every case - simple rinsing will remove all measurable
levels of enzyme from the paper.
Further investigation using a Perkin-Elmer Lambda 3B UV-vis
spectrophotometer with surface absorption accessory reveals that
rinsing protocols typical of those practiced in the conservation
community remove 30% or less of the enzyme bound to the paper.
Generally speaking, it was found that two-minute washes with
straight ethanol were consistently the least effective at removing
amylase residues left after in situ denaturation, removing only 18-
20% of the denatured protein residues adhered to the paper.
Similarly, two-minute washes with 50% aqueous ethanol (v/v) removed
18-29% and with distilled water removed 27-30%. In each case, the 1
cm x 2 cm Whatman #1 Chromatography (W1C) paper samples rinsed were
pre pared by a five-minute soak in an 0.1% (w/v) a-amylase solution. This was followed by a
two-minute in situ denaturation by addition of 50% (v/v) ethanol,
finishing with a double blotting. The strips were then rinsed with
gentle agitation in 10 ml of the above described wash baths. The
strips were again double blotted, and were permitted to air dry.
They were then subjected to surface UV-vis absorption
spectrophotometry. Kubelka-Munk transformation of the resulting
spectra was regarded as unnecessary because of the thinness of the
W1C paper.
THE BOTTOM LINE:
ACKNOWLEDGEMENTS:
The author is deeply indebted to David Laude and Linda Davis,
both of whom arranged access to essential equipment, as well as to
Karen Pavelka, James Stroud and Olivia Primanis, without whose
encouragement and support the work would never have passed outside
the walls of the HRHRC.
CITATIONS AND NOTES:
1Technically, amylases catalyze the
hydrolysis of a-D-1,4 glycosidic bonds between a-D-glucopyranose units.
An excellent review of the technical details of amylase chemistry
can be found in J.F.Robyt, "Enzymes in the Hydrolysis and Synthesis
of Starch," in Starch: Chemistry and Technology, 2nd Ed., ed.
by R.L. Whistler, J.N. Bemiller and E.F. Paschall, (Orlando: 1984,
Academic Press).
2Pintauro, N.D., Food Processing
Enzymes: Recent Developments. (Park Ridge, NJ: Noyes Data
Corporation, 1979). pp.93-148.
3Johnson, J.C., Industrial
Enzymes: Recent Advances. (Park Ridge, NJ: Noyes Data
Corporation, 1977). pp.148-215.
4Kulp, K.; Carbohydrases, in Food
Enzymes in Food Processing, 2nd Ed, ed. by G. Reed. (New York:
Academic Press, 1975.) pp. 54-122.
5Reed, G. Effective temperature and
pH, Enzymes in Food Processing, 2nd ed. Ed. by G. Reed, (New
York: Academic Press, 1975.) pp.31-42.
«« end of B&PGA 11: p. 32 »»
6Gould, B.J., Enzyme data, in
Handbook of Enzyme Biotechnology, ed. by A. Wiseman. London:
Ellis Horwood Limited, 1975, pp. 128-162, esp. carbohydrases on pp.
134-145.
7Wiseman, A., Industrial practice
with enzymes, in Handbook of Enzyme Biotechnology, ed. by A.
Wiseman. London: Ellis Horwood Limited, 1975, pp. 243-272, esp.
carbohydrases on pp. 243- 247 and tables.
8Daniel, R.M., Bragger, J., Morgan,
H.W.; Enzymes from extreme environments, in Biocatalysis, ed.
by D.A. Abramowicz. (New York: Van Nostrand Reinhold, 1990). pp.
243-254.
9Zeikus, J.G., Lowe, S.E., Saha,
B.C.; Biocatalysis in anaerobic extremophiles, in
Biocatalysis, ed. by D.A. Abramowicz. (New York: Van
Nostrand Reinhold, 1990). pp. 255-276.
10Pedersen, S. and Norman, B.E.;
Enzymatic modification of food carbohydrates, in Chemical Aspects
of Food Enzymes: The proceedings of a symposium organized by the
food chemistry group of the industrial division of the Royal Society
of Chemistry, ed. by A. T. Andrews. (London: Royal Society of
Chemistry, 1987). pp.156-187.
11Aunstrup, K., Production,
isolation, and economics of extracellular enzymes, in Applied
Biochemistry and Bioengineering, Vol. 2: Enzyme Technology, ed.
by Wingard, L.B., Katchalski-Katzir, E., Goldstein, L. (New York:
Academic Press, 1979), pp. 27-69.
12Thoma, J.A., Spradlin, J.E., and
Dygert, S; Plant and animal amylases, in The Enzymes, 3rd Ed.,
Vol. V: Hydrolysis (Sulfate Esters, Carboxyl Esters, Glycosides)
and Hydration, ed. by P.D. Boyer. 1971: Academic Press, New
York, pp. 115-189. A classic review of the a-amylases of mammals and higher plants.
13Takagi, T., Toda, H., Isemura, I.;
Bacterial and mold amylases, in The Enzymes, 3rd Ed., Vol. V:
Hydrolysis (Sulfate Esters, Carboxyl Esters, Glycosides) and
Hydration, ed. by P.D. Boyer. 1971: Academic Press, New York,
pp. 235-271. The classic review of the microbiological a-amylases.
14G. Bosch, J. Carswell and G.
Petherbridge, Islamic Bindings and Bookmaking, The Oriental
Institute Museum, The University of Chicago: pp. 35-36.
15J. Segal and D. Cooper, The Use of
Enzymes to Release Adhesives, The Paper Conservator: Journal of
the Institute of Paper Conservation, 1977, 2: 47-50.
16I am grateful to Dr. Greg Wall,
Sigma Chemical Company, for the initial suggestion that a test of
this sort be developed.
17A humid oven may be accomplished
by maintaining a water level in a pan whose surface area is equal to
about half the floor area of the oven. No great harm is inflicted
if the pan goes dry occasion ally, but the most effective
cross-linking will take place in a relatively humid environment.
The adhesive may be considered intractable when it no longer
releases easily after a brief immersion in water.
18Cooper, D., King, C., and Segal,
J., The use of enzymes in partially non-aqueous media, in The
Conservation of Library and Archive Materials and the Graphic Arts:
Abstracts and Preprints of the International Conference on the
Conservation of Library and Archive Materials and the Graphic
Arts, ed. by G. Petherbridge, (Cambridge: 1980, Institute of
Paper Conservation and The Society of Archivists), p. 14-19.
19Kieboom, A.P.G., Enzymes that do
not work in organic solvents: Too polar substrates give too tight
enzyme-product complexes, in Biocatalysis, ed. by D.A.
Abramowicz. (New York: Van Nostrand Reinhold, 1990). pp.
357-364.
20Full details of the investigations
are reported in a forthcoming monograph or book from researchers led
by H. Burgess at the Canadian Conservation Institute, Ottawa. The
results have been verbally communicated at several venues and are
widely accepted. H. Burgess, personal communication.
21Wakim, J., Robinson, M. and Thoma,
J.A.; The active site of porcine pancreatic a-amylase: Factors contributing to catalysis,
Carbohydrate Research 10, 1969, pp. 487-503, esp. p.
494.
22Toda, H. and Narita, K.,
Replacement of the essential calcium in Taka-amylase A with other
divalent cations, Journal of Biochemistry (Tokyo) 62,
1967, pp. 767-768.
23Prices for manually operated
micropipettes range from about $65-$225 ($US, 1992), depending on
features, precision, accuracy and service life. The price-conscious
occasional user might with reasonable confidence select a
micropipette like those described by Cole-Parmer as Low Cost
Pipettes, Adjustable Volume, Cats. No. L-24800-00 (10-50 µl)
and L-24800-10 (50-200 µl), along with a box of their
corresponding disposable tips, Cat. No. L-07953-20 (960 non- sterile
pipette tips, 1-200 µl, prepacked in 10 dispensing racks).
Alternatively, users affiliated with a university may wish to borrow
a micropipette of the appropriate volume range from a research group
in the biological or biochemical sciences.
24Do not contaminate the bulk liquid
by attempting to measure its volume directly. Instead, estimate the
volume visually, perhaps by filling a comparable-sized bottle to the
same level and measuring the volume of this substitute sample. For
the purposes of enzyme preparations, an accuracy of ±25%
should suffice in any treatment.
25Dr. Greg Wall, Sigma Chemical Co.
Technical Support, telephone communication.
26Anyone extending these principles
to proteases should definitely freeze premeasured aliquots of
proteases, because refrigerated samples are subject to
autodigestion.
27Segal and Cooper, 1977, op
cit., p. 47.
28personal communication, June 3,
1992.
29Boel, E., Brady, L., et al., 1990.
Calcium binding in a-amylases: An x-ray
diffraction study at 2.1-Å resolution of two enzymes from
aspergillis, Biochemistry 29, 6244-6249.
30Thoma, J.A., Spradlin, J.E.,
Dygert, S; 1971, op cit., p. 133-4.
31Wakim, J., Robinson, M. and Thoma,
J.A.; 1969, op cit.
32Boel, E., Brady, L., et al, op
cit.
33Lot 50H0607 was labeled by Sigma
as containing 1400 units of activity/mg solid and 1900 units/mg
protein, where the second figure is merely the result of a Biuret
assay of the solid's protein content. Sigma defines a unit of
activity as the liberation of "1.0 mg of maltose from starch in 3
minutes at pH 6.9 at 20°C" for most of their a-amylases (Sigma Co. catalog, 1990-91).
34J. Segal and D. Cooper, 1977,
op cit. While Segal and Cooper used an 0.1%(w/v) solution of
a-amylase dissolved in a 10%(v/v)
solution of ph=7.2 phosphate buffer, informal polling of
conservators indicated that essentially every bench conservator
using enzymes was doing so with a solution in straight distilled
water. The experiments reported in this article were intended to
quantify residues left under treatment conditions actually in use,
and were consequently executed with simple aqueous 0.1%(w/v) amylase
solutions.«« end of
B&PGA 11: p. 34 »»