
Reilly, James M. The Albumen & Salted Paper Book: The history and practice of photographic printing, 1840-1895. Light Impressions Corporation. Rochester, 1980.
| [Previous] Chapter 8 | Title Page | [Next] Chapter 10 |
| Table of Contents | Search this book |
The permanence of prints on albumenised paper and their freedom from yellowness with age undoubtedly depend on the elimination, by the fixing bath, of the insoluble salts of silver.
--A. Haddon and F.B. Grundy July, 18971
This chapter deals with two important operations in the processing of albumen and salted papers. The first is the fixing step, in which the unexposed light-sensitive substances are removed from the prints by treatment in a solution of sodium thiosulfate. The second and equally important step is the washing out of the chemical products of the fixation process. These must be removed because they are unstable and will cause yellowing and fading of the image if allowed to remain. Washing prints in running water and treatment in a "washing aid" such as a 1% sodium sulfite solution or Kodak Hypo Clearing AgentTM are the means employed to remove the by-products of fixation.
While the broad outlines of the fixing and washing operations with albumen and salted papers are similar to those of other photographic materials, there are some significant differences. Because of the extremely small size of the silver image particles in these papers, the image is considerably more vulnerable to chemical attack, especially from the residual products of fixation. Therefore it cannot be over-stressed that strict adherence to correct procedures in fixing and washing is the only way to assure optimum permanence in albumen and salted paper prints.
Theory of the Fixation Process
In an exposed but unprocessed print the image layer consists of the metallic silver image itself, the binder material used (starch, gelatin, albumen, etc.) and unexposed light-sensitive silver salts. These are primarily silver chloride, but depending on whatever additions have been made to the salting or sensitizing solutions, silver citrate, silver chromate or other silver salts may be present. In order to render the image stable the light-sensitive substances must be removed, ideally leaving behind only the binder material and a silver (and gold or platinum, if the print has been toned) image.
Silver chloride and the other silver salts are not soluble in water; they can only be removed through a chemical reaction with another substance, in which a new water-soluble "complex" is formed. The choice of fixers or more properly, "complexing agents" for silver salts--is somewhat limited. Among the substances which have some fixing or stabilizing action are ammonia, potassium cyanide, strong chloride solutions, thiocyanates, thiourea, sodium sulfite and sodium and ammonium thiosulfate. Of all these sodium thiosulfate has the fewest drawbacks for the purpose of fixing silver printing-out papers, and it has been in almost exclusive use for that task since the very earliest days of photography.
The discovery of the fixative properties of sodium thiosulfate (or "hyposulfite of soda" as it was known then) was made in 1839 by Sir John F. W. Herschel. He had heard that both Talbot and Daguerre had evolved photographic processes, and decided to make some investigations himself. He first applied sodium thiosulfate for the purpose of fixing silver chloride papers on January 29, 1839. The idea came to him because of some observations he had made in the years 1819 and 1820, when he discovered the substance sodium thiosulfate and noticed that it was a solvent for silver chloride.2 A short while later he communicated his epochal discovery to Talbot, who up to this point had not been truly fixing but merely "stabilizing" with a strong solution of sodium chloride. Talbot at first disdained "hypo," but later incorporated it into his own process, as did Daguerre, who had also been "stabilizing" with a strong sodium chloride solution (in a stabilizing treatment the silver chloride is not removed, it is merely changed into a less sensitive form).
Sodium thiosulfate in its pentahydrate form has the chemical formula Na2S2O3�5H2O. Each thiosulfate ion contains 2 atoms of sulfur, and it is chiefly the presence of sulfur that necessitates the removal of the silver thiosulfate complexes which are the products of the fixation process. Also, any thiosulfate which has not "complexed" with silver must be removed as well, because the thiosulfate ion is itself unstable and can be easily decomposed, thus releasing elemental sulfur to attack the silver image. However, in dry crystalline form sodium thiosulfate is a stable substance, although it should be kept in a tightly stoppered bottle. When dissolved in water, sodium thiosulfate is not stable, and after a time partially decomposes to sodium sulfite and elemental sulfur. For this reason fixing solutions intended for use with albumen and salted paper prints should not be made in advance; the fixer solution should preferably be made up just prior to use, so that spontaneous decomposition of the thiosulfate is kept to a minimum.
The actual reactions involved in the fixing process are fairly complicated, and there are probably at least three different kinds of silver-thiosulfate complexes formed during fixation.3 Studies of these reactions have led to the conclusion that certain conditions must prevail for the most effective fixation as well as for the most complete removal of free thiosulfate and silver-thiosulfate complexes. The most important of these conditions is that thiosulfate ions must be present in excess. There must be more thiosulfate ions present than are needed to react with all the silver ions present, or else insoluble complexes are formed which cannot be washed from the image layer.4 Another way of stating this is that one of the complexes formed during fixation is only soluble in fresh thiosulfate.
In practice this means that two fixing baths are necessary; the first one does the bulk of the "complexing," while the second insures that the complexes ultimately formed can be washed out of the print. It is apparent from this why only fresh fixer solutions are likely to result in optimum print permanence. As the fixer solution approaches a point of exhaustion, it loses its ability to form soluble silver-thiosulfate complexes. Studies have also shown that the fixing process is completed fairly rapidly, and prolonged fixation is much more injurious to prints than is generally believed. Fixing too long will allow thiosulfate to penetrate inside the paper fibers, in which case it becomes almost impossible to remove.
Another potential difficulty arises from the pH of the fixing bath. Although modern gelatin-based photographic materials are generally fixed in an acid hardening type of fixing bath, albumen and salted paper prints are best fixed in an alkaline solution of sodium thiosulfate to which no hardeners have been added. Alkaline thiosulfate solutions are necessary for two reasons: first, the slight alkalinity prevents any acids which might be inadvertently introduced into the fixing bath from decomposing the sodium thiosulfate and liberating sulfur. Second, an acid fixing bath would tend to attack the finely divided metallic silver of the image, causing excessive bleaching of the highlights and middletones in the print. This attack on the image silver itself is minimized when the pH of the fixer is kept on the alkaline side.
The Practice of Fixation
Based on the theoretical considerations given above, fixation of albumen and salted papers is best done in a freshly made-up solution composed as follows:
| Sodium thiosulfate (pentahydrate) | 150 g |
| Sodium carbonate | 2 g |
| Water | to make 1 liter |
The fixing bath should be made with water slightly warmer than the desired working temperature of 18 to 200 C, since some heat is always consumed in the formation of the solution. It is important that the fixing bath not be too cold, since too great a temperature differential between the fixing bath and the other processing solutions might cause blistering of albumen paper. To insure that fixation is properly carried out, the prints must be washed free of other substances--especially acidic platinum toning baths--before they are placed in the fixer. Two separate trays of fresh fixer are required. The prints are placed in the first fixing bath and agitated constantly for 4 minutes, drained for at least 5 seconds, then placed in the second fixing bath and agitated constantly for another 4 minutes. It is important that there be enough fixing solution in each tray to easily accommodate the number of prints being fixed at one time, and that care be taken to see that the whole surface of each print is constantly washed over with fixer solution. As indicated in Chapter 3, this fixing procedure is applicable to all silver printing-out papers.
It is difficult to establish the point at which a fixing bath becomes exhausted. The value of maximum solubility of silver chloride in sodium thiosulfate is known, but that does not help to establish the practical limits of use for a fixing bath, since the presence of excess thiosulfate is required to insure maximum permanence. Estimates in the literature suggest that no more than 150 8 x 10 inch prints should be fixed in 1 liter of 15% sodium thiosulfate solution,' but it seems that a much more conservative estimate should be made when optimum permanence is desired. To assure the longest life for a print, probably no more than 10 to 15 prints (approximately 8 x 10 inches in size) should be fixed in each liter of fixer solution.
The silver content of printing-out papers is very high relative to conventional develop-out materials, and it can also vary from one kind of albumen or salted paper to another. The relatively high silver content and the vulnerability of the colloidal silver image are the reasons why such a conservative estimate of fixer exhaustion is necessary. Compared to the other costs of albumen and salted paper printing, the price of fixer is cheap, and frequent renewal of the fixing bath is a worthwhile investment which helps insure durability of the finished prints.
During fixation the prints undergo a dramatic change in color, in which the original brilliant purple or brown color is transformed into a much yellower and duller brown hue, accompanied at the same time by a loss of density. The reasons for this color and density change--a characteristic phenomenon in all silver printing-out papers--have to do with the physical structure of the metallic silver which forms the image. In an exposed but unfixed print the silver image particles exist in a highly dispersed state, forming a kind of "solid solution" of metallic silver in silver chloride.6 The color and density of this system is determined by the size and amount of metallic particles as well as the combined refractive indices of silver chloride and whatever binder materials have been used on the paper.
Upon fixation the silver chloride is removed and the metallic silver particles undergo a "packing" process and accumulate into aggregates of particles.7 At the same time the refractive index of the system is lowered by the removal of the silver chloride. Together these physical and chemical changes cause a loss of particle covering power i.e., print density and shift the color of the print toward yellow. In fact, any physical change in the image layer causes a shift of print color. A good example of this is the reddening of prints that occurs when they are placed in the initial wash water; swelling of the image layer is the actual cause of the color shift. When prints dry they undergo another change, becoming more neutral in color and gaining slightly in density as the image layer contracts.
The purpose of washing is to remove the sodium thiosulfate and silver thiosulfate complexes that remain in the print after fixation. Over the years a great deal of attention has been paid to the theory and practice of washing photographic materials, but insufficient washing still remains a leading cause of the deterioration of photographs. The washing of photographic prints is more difficult than the washing of films, primarily because of the absorption of thiosulfate into the paper fibers. With prints the rate of washing slows down tremendously at the lower levels of thiosulfate concentration,8 and in practice it is impossible to remove every trace of thiosulfate simply by washing in water.
In the case of albumen and salted papers, effective washing is even more important than in modern photographic materials. In the older papers, the silver image is in much more intimate contact with the paper fibers than is the case with modern papers, where a substratum of baryta and gelatin (or polyethylene) separates the image layer from the paper base. Therefore, with albumen and salted papers a combination of both proper fixation and effective washing is necessary to insure that the base paper does not become a reservoir of image-threatening substances. It is very important in this regard that immersion of the prints in the fixing solution is not prolonged beyond the recommended time. Experimental evidence on the washing of albumen and salted paper prints is almost nonexistent. However, the factors which affect the rate of washing gelatin prints are probably valid for these papers as well.

Fig. 38. A semi-automatic print washer and agitator, Ca. 1890.
The removal of thiosulfate from prints cannot be accomplished simply by washing in water, if a print of optimum stability is desired. In fact, water is such a poor remover of low levels of thiosulfate from prints that an extra step in processing is needed to assure maximum print permanence. This consists of treating the prints in a so-called "washing aid" or hypo clearing agent in order to facilitate more complete removal of the thiosulfate. These treatments are effective because they displace the absorbed thiosulfate ions and replace them with less harmful and more soluble ions of various salts. The best "washing aid" for albumen and salted papers consists of a 1% solution of sodium sulfite, although proprietary formulas such as Kodak Hypo Clearing Agent may also be used.
The actual apparatus and conditions of washing are very important to the efficiency of the washing process. In general the desirable factors in the design of washing apparatus are assurances that sufficient flow of water covers the entire print surface, and that the entire volume of water is changed at least every 5 minutes. The temperature of the wash water should remain fairly constant at approximately the same temperature as the other processing solutions. Where the design of the washing system is less than perfect--for example when more than 2 prints are washing in a tray equipped with a tray siphon--hand agitation of the prints is absolutely necessary. Another common washing apparatus is merely a round tub with a water inlet that forces the prints to swirl in a circle. This kind of washing apparatus also demands frequent hand agitation to insure proper washing.
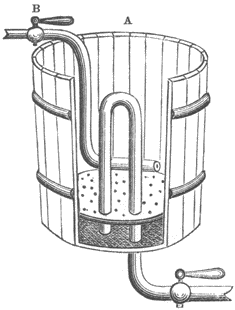
Fig. 39. A print washing tank with a circular water flow and siphon drain, ca. 1870.
Certainly the average conditions for washing prints in the 19th century were less than optimum. Often the prints were washed in ice cold water, or without the benefits of running water of any kind. The basic technique recommended in many manuals of the time was to remove the prints from the wash tray, squeegee them, then return them to a fresh tray of water and agitate them. This procedure was to be repeated over a period of time that ranged from 12 hours (according to Abney)9 to only 15 minutes (according to Haddon and Grundy).10 Nineteenth-century photographers were very much concerned with assuring removal of thiosulfate, and then as now, there were all sorts of washing devices and hypo-elimination preparations on the market.
The washing time of prints depends on the thickness of the paper base and the nature of the binder material used. Generally, the thicker the base paper, the longer the wash time required, and also the longer the treatment time required for the "washing aid." The removal of thiosulfate is also affected by the diffusion rate of substances through the image layer, so that albumen paper will probably require substantially longer to wash than papers like arrowroot or plain salted paper that possess more porous image layers. The wash times recommended below are intended to be safe for glossy albumen paper and all other salted papers as well. Some papers, however, may be injured by prolonged washing--the image layer may begin to dissolve--in which case a shorter wash time is indicated.
METHOD FOR WASHING ALBUMEN AND SALTED PAPERS
After fixing is complete the prints should be given a short (2 to 4 minute) wash in running water before treatment in the "washing aid." This water wash removes the vast bulk of the thiosulfate and avoids overloading the mechanism of ion-exchange in the sodium sulfite solution to follow. The prints are then to be treated for 3 to 4 minutes with constant agitation in the following solution:
| Sodium sulfite (anhydrous) | 10 g |
| Water | to make 1 liter |
This constitutes a 1% solution. It should be used only once and discarded. The solution may be made in advance. The rate of exhaustion for this solution is the same as that of the fixer solution--no more than 20 prints, approximately 8 x 10 inches, should be treated in 1 liter of solution.
Following the sodium sulfite bath the prints should receive 30 minutes of washing in running water in an effective print washer. Extremely heavy base stocks and very thick coatings of albumen may indicate that a longer wash time--40 to 50 minutes--is required.

Fig. 40. A drying rack for prints. The prints were not allowed to become truly dry, but were mounted while still damp.
After the wash step is completed, the prints should be removed from the print washer and either gently squeegeed, face-down on clean glass, or blotted with photographic quality blotters to remove excess water. They should then be air dried face-up on clean fiberglass screens or on suitable blotters. The degree of curling in the dried prints depends on the thickness of the paper base and the nature of the binder material used. Thin papers coated with heavy layers of albumen tend to curl the most --hence the almost universal historical practice of mounting albumen prints while still damp. Prints may be dried under weights or in a book press to help prevent curling; in this case the coated side of the print should be against silicone release paper, while blotters should be placed against the back side of the print.
| [Previous] Chapter 8 | Title Page | [Next] Chapter 10 |
| Table of Contents | Search this book |