PREVENTIVE CONSERVATION RESEARCH AND PRACTICE AT THE BRITISH MUSEUMSUSAN BRADLEY
4 REDUCED SULFUR GASESThe reduced sulfur gases hydrogen sulfide (H2S) and carbonyl sulfide (COS) are important because they form silver sulfide on the surface of silver objects causing them to tarnish, form black sulfides on copper alloy objects, and react with lead pigments causing blackening. There is no need for any material which emits these gases to be used in the storage or display of collections since they can be easily identified during the accelerated corrosion test. However even though only materials which do not tarnish silver are used in the Museum, reduced sulfur gases are present in the external air at concentrations of parts per trillion and are present in the unfiltered air in the Museum. Very low concentrations of these gases can cause silver to tarnish. In the British Museum research into these gases has focused on the tarnishing of silver since this has such an impact on conservation effort and time, with many objects requiring regular cleaning. Silver used to be lacquered to reduce the rate of tarnishing, but removing lacquer was difficult and residues have recently been shown to promote tarnishing (Thickett and Hockey 2003). Another approach was to use silver cleaners which included tarnish inhibitors, usually mercaptan based (Wilthew 1981, 1987). 4.1 TESTING TARNISH PREVENTION PREPARATIONSBetween 1975 and 1987 different types of tarnish-prevention preparation were tested for use in the Museum. The preparations included vapor-phase inhibitors, protective papers and cloth for wrapping or enclosing silver, and absorbent materials. The testing took the form of exposing a cleaned silver coupon and the material under test in a closed container with a source of hydrogen sulfide. Other researchers used either artificially high levels of hydrogen sulfide or carbonyl sulfide (Franey et al. 1985). The effectiveness of the preparations was determined by the number of days taken for tarnish to be visible on the silver coupons. In these tests all of the preparations retarded the formation of tarnish when compared with the time taken for the control coupons to tarnish. However the most effective preparations were 3M silver protector strip, Tarnprufe cloth, four ICI catalysts, of which 75-1 was most effective and Charcoal Cloth (Bradley 1983; 1985; 1989; Duncan 1986b; 1987). As 3M protector strip was designed to be used in smaller closed storage boxes and Tarnprufe cloth, which contained zinc acetate as a sulfide scavenger, was designed to enclose silver, neither was suitable for use in the display of silver objects. ICI catalyst 75-1, a highly porous zinc oxide (wurzite) bonded with cement, designed to remove hydrogen sulfide from North Sea gas, and Charcoal Cloth were the most promising preparations for use in showcases to reduce or remove reduced sulfur gases. In 1993 a more rigorous investigation of silver tarnishing to quantify the efficiency of the tarnish inhibitors and identify the nature of silver tarnish in the Museum began (Lee 1996). The high hydrogen sulfide concentrations used in previous experiments formed a tarnish layer on silver was very much easier to remove than that formed naturally in the Museum galleries and in the laboratory air. As a result it was decided to use concentrations of reduced sulfur gases as near to ambient levels as possible. There was a precedent for conducting corrosion experiments with silver at naturally occurring levels of hydrogen
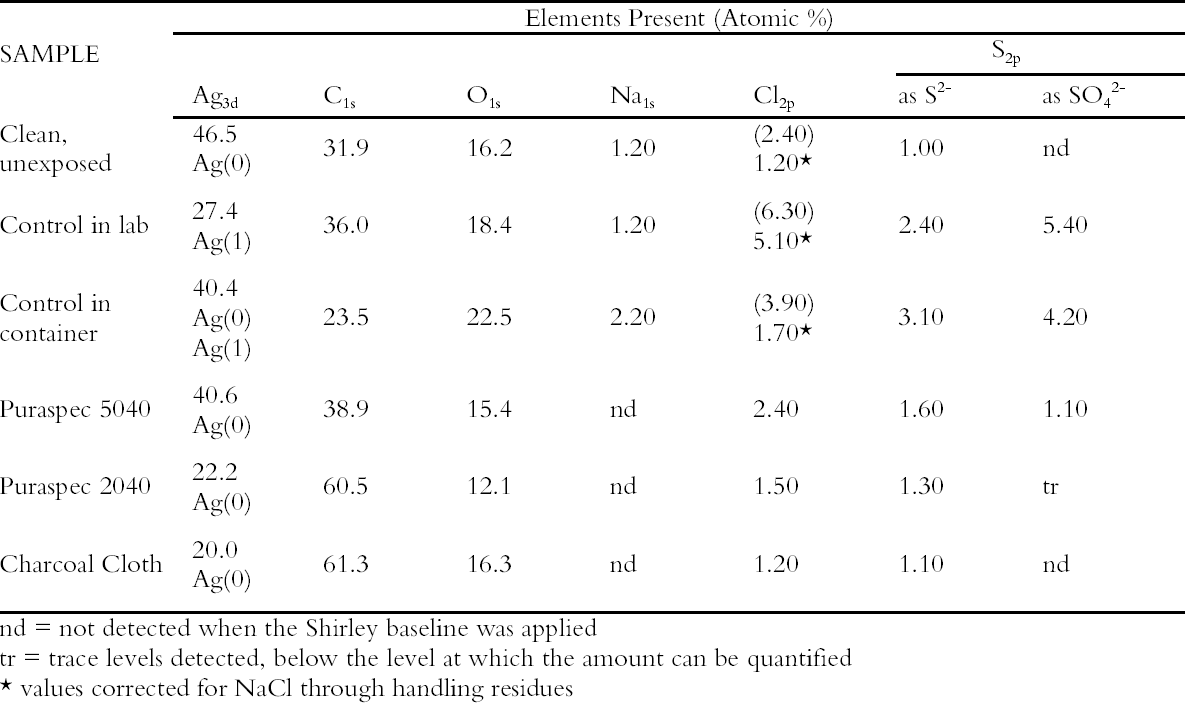
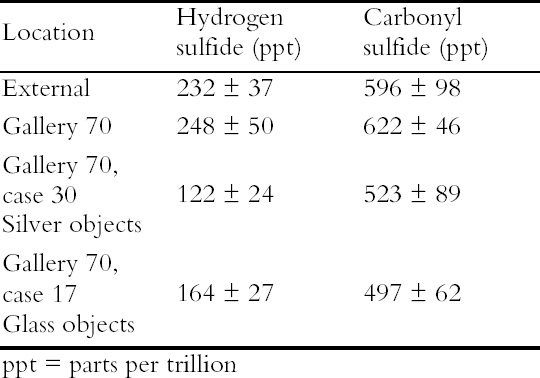
A small aquarium pump was used to pump air through a series of 2L capacity containers. Two holes were drilled into the screw tops of the containers, one for the inlet tubing and one for the air outlet. The containers were connected by rigid Teflon tubing and polythene T-connectors. The arrangement ensured that the pathway to each of the containers was exactly the same length, volume and had the same number of joins and that each container received an equal air flow. Eleven materials were evaluated in this experiment but only the results on the two most effective ICI catalysts, now named Puraspec 5040 and 2040, and on Charcoal Cloth are reported here (Table 1). The materials were placed in individual containers with the cloth cut to cover the base and the Puraspec pellets added to form a monolayer. Analar silver coupons were cleaned by abrasion and degreased in high purity acetone before being pierced and weighed. One coupon was then suspended from the screw top of each container, all at the same height. Controls were set up with a silver coupon suspended in a container alone and one suspended in the area of the apparatus in the laboratory. The experiment was started in July 1994 and completed in April 1995. When the experiment was stopped the control in the laboratory was a dark yellow brown color and that in the container was distinctly yellowed. The silver coupons exposed with the Charcoal Cloth and Puraspec 5040 and 2040 showed no visible change, but at 60x magnification, black spots could be seen. 4.1.1 Analysis of Silver Test CouponsPrior to analysis the coupons were individually stored in bags made from a film with low permeability to gases along with sachets of Ageless oxygen scavenger to prevent further change. The coupons were analyzed by X-ray photoelectron spectroscopy (XPS) with a Kratos XSAM series 800 using Mg and Al Kα radiation at 300 W using 7.5mm slits and 80/40 eV pass energies. The results in Table 1 (Johnson Matthey Technology Centre 1995) show that the silver tarnish contained oxides, chlorides, and sulfates in addition to the expected sulfide. On the control coupons, sulfide was present at lower concentrations than oxide and sulfate. Despite efforts to minimize handling of the samples the presence of sodium suggested handling contamination on some of the coupons and an 4.2 REDUCED SULFUR GAS MONITORINGAs part of a broader pollutant gas monitoring campaign in the Museum, hydrogen sulfide and carbonyl sulfide levels were monitored using diffusion tubes supplied by S. Watts of Oxford Brookes University, Oxford, UK. The tubes and silver coupons were deployed in Gallery 70, Rome City and Empire, in the gallery, in two showcases containing silver objects, in a control case containing only glass, and outside of the Museum building for 28 days. The showcases were different sizes but were constructed to the same specification, and the case inserts, dressing fabrics and label materials had been tested to ensure that they did not out-gas reduced sulfide gases. The analysis of reduced sulfur gases was interesting, as carbonyl sulfide was present in greater concentration than hydrogen sulfide at all of the locations monitored. The levels inside showcases were almost as high as the levels in the gallery and externally (Table 2). Only the coupon exposed externally was visibly tarnished, particularly at the edges. Taking the measurement
These coupons were analyzed by XPS and Static Secondary Ion Mass Spectrometry (SSIMS). The SSIMS analysis was carried out using a Millbrook Chemical Microscope in large area (2.25 mm2), static mode for both negative and positive ions. The primary beam is provided by a raster scanned gallium liquid metal ion gun with low energy optics for secondary ion extraction into a 300 Da quadropole mass spectrometer. Although both methods are surface analysis techniques the information they provided is different. In XPS the elements present are separated according to their binding energy (eV) and hence sulfur as sulfide and as sulfate are discriminated. The analysis is quantified as atomic concentration percent. SSIMS is a non-quantitative technique and the output is a mass spectrum detecting fragments as well as ions. Sodium, chlorine, oxygen, zinc, sulfur as sulfide and as sulfate, silicon, and carbon were detected on the surface of the silver coupons. 4.3 THE NATURE OF SILVER TARNISHIn this program of monitoring, the nature of silver tarnish in the British Museum was confirmed as a mixture of oxide, chloride, sulfate, and sulfide. Carbon was again a major contaminant on the surface of the coupons with fragments of up to C6 in chain length present as determined by SSIMS. In this work, handling of the coupons was dramatically reduced by mounting them on aluminum stubs for presentation in the SSIMS equipment and storage in aluminum containers prior to exposure and analysis. Hence carbon species in the atmosphere were indicated as the primary source of this contamination (Hallett et al. 2003). Carbonyl sulfide was shown to be the predominant reduced sulfur gas in the air and not hydrogen sulfide; hence, the strategy of basing a tarnish prevention system on a material designed to remove hydrogen sulfide was flawed. 4.4 GALLERY TRIALSMeanwhile the evaluation of the passive deployment of Puraspec 5040 was continued in gallery trials. The pellets were deployed inside a large Lshaped showcase displaying silver objects and were visible. Silver coupons were put into this case and
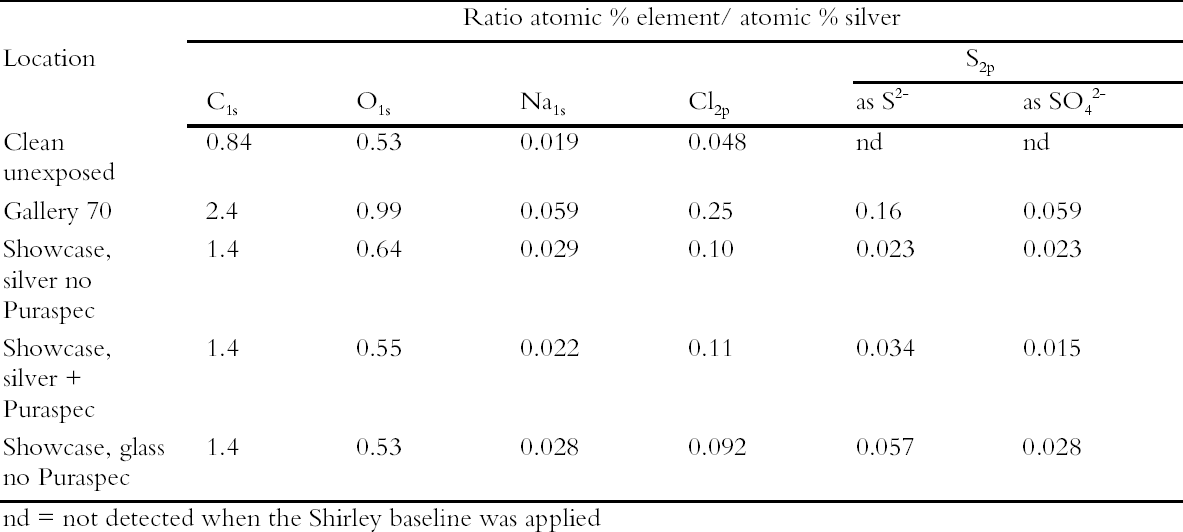
The coupons in the control locations tarnished after 42 days. All of the coupons were then analyzed by XPS. The results are presented in Table 3 as ratio atomic percent. There was more sulfur present on the silver coupon exposed in the showcase containing glass objects than that containing silver objects and no Puraspec. Hence the trial showed that silver objects are likely to tarnish at a slower rate in showcases containing other silver objects. This is presumably because the silver objects act as a sink for reduced sulfur and other gases. The Puraspec 5040 in passive mode did not reduce the rate of tarnishing. The results of this experiment were later found to have been affected by the high air exchange rate of the complex L-shaped showcase, more than 8 air changes per day. A trial of a positive pressure system was undertaken replacing Puraspec 5040 with Puraspec 2030 which was suggested by ICI Katalco. This product is also formed from zinc oxide and contains aluminum oxide which oxidizes carbonyl sulfide to hydrogen sulfide which is in turn absorbed by the zinc oxide, and a copper compound which acts as an indicator, turning from green to black when it takes up sulfides. A simple filter bed was prepared in a tubular container using Puraspec 2030 and activated carbon cloth. It was decided to incorporate the cloth since it absorbs a broad range of pollutant gases and pumping air into a case could lead to a build up of pollutants at the surface of the objects. The filter bed was connected through the base of a showcase displaying only silver by plastic tubing and a small pump. Silver coupons were deployed to monitor the rate of tarnish formation and these were visually tarnished after 60 days. Again they were analyzed by XPS which showed that the pump had been effective at reducing sulfide formation (Table 4). On the basis of this research, current thought is to use positive pressure systems in all showcases where silver objects are displayed. |