PREVENTIVE CONSERVATION RESEARCH AND PRACTICE AT THE BRITISH MUSEUMSUSAN BRADLEY
ABSTRACT—Since the early 1970s the conservation scientists at the British Museum have pursued a program of research in what has become known as preventive conservation. This object-centered research almost always was initiated as investigation into the cause of deterioration, to gain an understanding of treatment and environmental requirements for stabilization. The majority of galleries and object storage areas are not equipped with air-handling systems; such systems are not always feasible in a building which is itself of such importance that it has been designated grade one listed by English Heritage. Solutions have thus involved micro-environments tailored to the needs of groups of objects. A continuing theme has been the role in deterioration played by pollutant gases, particularly reduced sulfide gases and organic acids. This paper presents an overview of these investigations in the broader context of preventive conservation practice in the British Museum. TITRE—Recherche et mise en pratique de la conservation pr�ventive au British Museum. R�SUM�—Depuis le d�but des ann�es 70 les scientifiques de la conservation du British Museum (mus�e national britannique) ont poursuivi un programme de recherche en conservation pr�ventive. Cette recherche centr�e sur les objets a presque toujours d�but� en tant qu ‘�tude sur les causes de la d�t�rioration, afin de mieux comprendre les n�cessit�s de traitement et les conditions ambiantes id�ales pour la stabilisation. La majorit� des salles d'exposition et de r�serve ne sont pas �quip�es de syst�mes de climatisation; de tels syst�mes ne sont pas toujours possibles dans un b�timent qui est lui-m�me d'une telle importance historique qu'il a �t� class� dans la cat�gorie de plus haute importance �tablie par English Heritage (h�ritage anglais). Les solutions ont ainsi impliqu� des micro-environnements con�us en fonction des besoins de groupes particuliers d'objets. Un th�me constant de la recherche a �t� le r�le jou� dans la d�t�rioration par les gaz polluants, en particulier les gaz sulfureux r�duits et les acides organiques. Cet article pr�sente une vue d'ensemble de ces investigations dans le contexte plus large de la pratique en mati�re de conservation pr�ventive au mus�e. TITULO—Investigaci�n y pr�ctica de la conservaci�n preventiva en el British Museum (Museo Brit�nico). RESUMEN—Desde principios de la d�cada de 1970 los cient�ficos de conservaci�n del British Museum (Museo Brit�nico) se dedican al �rea de investigaci�n conocida como conservaci�n preventiva. Esta investigaci�n centrada en los objetos comenz� como una b�squeda de las causas de deterioro para entender mejor los tratamientos y requisitos ambientales adecuados para su estabilizaci�n. La mayor�a de las galer�as y �reas de almacenamiento no est�n equipadas con sistemas de aire acondicionado; estos sistemas no son siempre factibles en edificios que por s� mismos son de tal importancia que han sido designados de grado uno en la lista de English Heritage (Patrimonio Ingl�s). As�, las soluciones han involucrado micro-ambientes ajustados a las necesidades de grupos de objetos. Un tema recurrente ha sido el rol de los gases contaminantes en el deterioro de los objetos, en particular el de los gases sulfurosos y los �cidos org�nicos. Este trabajo presenta una visi�n general de estas investigaciones en el contexto m�s amplio de las pr�cticas de conservaci�n preventiva en el British Museum (Museo Brit�nico). T�TULO—Pesquisa e pr�tica em conserva��o preventiva no British Museum (Museu Brit�nico). RESUMO—Desde o in�cio da d�cada de 1970 os cientistas de conserva��o do British Museum (Museu Brit�nico) t�m seguido um programa de pesquisa que ficou conhecido como conserva��o preventiva. Esta pesquisa baseada no objeto foi quase sempre iniciada pela investiga��o sobre as causas de deteriora��o, a fim de compreender o tratamento e as necessidades ambientais necess�rios para a estabiliza��o. A maioria das galerias e �reas de armazenagem de objetos n�o est�o equipadas com sistemas de controle de ar; tais sistemas nem sempre s�o vi�veis em edif�cios, os quais por si s� t�m tamanha import�ncia que s�o classificados como grau um na listagem do English Heritage (Patrim�mio Ingl�s). Por conseguinte, as solu��es envolvendo micro-ambientes s�o desenhadas � medida das necessidades de grupos de objetos. Um tema constante tem sido o papel dos gases poluentes, em particular dos gases sulf�ricos e �cidos org�ncios, na deteriora��o. Este artigo apresenta uma vis�o geral destas investiga��es no contexto mais amplo da pr�tica da conserva��o preventiva no British Museum (Museu Brit�nico). 1 INTRODUCTIONThe importance of the interaction of environment and collections was recognized long before the then Keeper of the British Museum Research Laboratory published The Conservation of Antiquities and Works of Art (Plenderleith 1956). This book contains a section on “The Influence of Environment” based on research on the deterioration of objects in the collection carried out by the Museum scientists between 1922 and 1950. By today's standards the information is rudimentary, but it did give useful guidance on collections care. In parallel to the work at the British Museum, research into the effects of the environment on oil paintings and on Japanese art was being carried out in other institutions. This work informed two important publications, The Museum Environment (Thomson 1978) and Characteristics of Japanese Art that Condition Its Care (Toishi and Washizuka 1987), both of which have contributed to the framework of what has become known as preventive conservation. In the early 1970s, under the leadership of A. E. A. Werner, British Museum conservation scientists began a concerted program of research into the effects of the environment on the collection. This research was driven by the development of new galleries and the need to establish whether or not control of temperature and/or relative humidity was needed to safeguard the objects, to justify its inclusion in the projects. There were several reasons not to attempt largescale climate control. First of all, being designated grade one listed by English Heritage means that the British Museum building is of such historical importance that it should be maintained in its original form. Putting in air conditioning for galleries without affecting the structure is very costly, aside from the expense of installation, running, maintenance, and replacement of the systems. Secondly, the trend was away from single-object galleries, such as the run of upper floor galleries displaying Greek pottery, to thematic mixed-object galleries where no one environment would be suitable for all of the objects. Finally, much of the collection is stable in the ambient conditions in the Museum and air conditioning was simply not needed. Hence, simple approaches and targeted control were favored for the display and storage of those objects which were found likely to deteriorate in normal ambient conditions. The research focused on single objects or groups of objects which were deteriorating, to identify the cause and a method of prevention. The role of relative humidity soon became apparent. Determining an appropriate relative humidity and suitable method of control was part of the prevention strategy. Gases given off by the materials used in storage and display, and sometimes given off by the objects themselves, were found to cause corrosion of metal objects and to form mixed salts on the surface of some porous stone and ceramic objects. In this paper an overview of the research between 1970 and 2005 on temperature, relative humidity, and pollutant gases is put in the context of the development of preventive conservation practice in the British Museum. 2 TEMPERATURE AND RELATIVE HUMIDITY2.1 COPPER ALLOY, WEEPING GLASS, IVORY AND BONE2.1.1 Copper AlloyIn the 1960s and '70s the emphasis of conservation in the British Museum was on the treatment of objects. Stabilization through treatments, such as soaking bronzes in sodium sesquicarbonate solution or stripping to remove corrosion layers, was seen as the appropriate response to deterioration. In the Museum Research Laboratory, studies into the deterioration of copper alloy artifacts had shown that bronze disease could be prevented by controlling the relative humidity to below 35%, stopping the reaction of nantokite with copper metal and moisture from the air to form paratacamite (Organ 1963). This research was implemented in the design of six new galleries opened between 1968 and 1975. Dehumidified showcases were incorporated to display Middle and Near Eastern copper alloy objects which were particularly prone to bronze disease. The showcases were controlled with desiccant dehumidifiers supplied by Munters Ltd. (Newey 1987). A dehumidified storage area was constructed for the copper alloy objects not on display. Although it took some time to limit the use of chemical stabilization methods to those objects for which a controlled environment could not be provided, control of relative humidity is now the primary method of preventing bronze disease. 2.1.2 Weeping GlassWeeping glass was shown to be stable if kept at an RH of 38-42%, and all of the weeping or crizzled glass was moved to a dehumidified showcase where the objects could be viewed without being handled (Organ and Bimson 1957; Werner 1958). This decision was based on the identification of potassium carbonate salts on the surface of the glass. At that time, analysis was restricted to those ions expected to be present, so it is not known whether some of the ions detected today on the surface of glass were present in the 1950s (Bimson 2004). The critical relative humidity for potassium carbonate was determined as 42%, above which it takes up moisture from the air, forming surface droplets which give the appearance of sweating and provide an aqueous medium into which more alkali ions can be leached from the glass. 2.1.3 Ivory and BoneThe collection contains ivory and bone objects from many different cultures of widely differing ages. Among the highlights of the collection are the Lewis chessmen, carved from walrus ivory and dated to AD 1150–1200. Some of the chessmen were found to be cracking and exfoliating and in 1970–71 all the pieces were examined by scientists working in Dental Anatomy at University College London, who identified the cause of the problem ( Boyd 1971). The chess pieces which were deteriorating had been carved in walrus ivory which had been boiled. This had caused denaturing which made the ivory more susceptible to cracking in response to changes in relative humidity. In 1978 the chessmen were exhibited in a showcase controlled by a ducted humidifier to 50–60% RH. Recently this has been redefined to 45–55% as the control achieved was always close to 50% and the chessmen remain stable. Ivory objects from Nimrud which had been burnt in antiquity are not humidified. They have been stable in an uncontrolled storage area that has naturally maintained 40–45% RH and have been seen to react adversely to higher and lower relative humidity. The most dramatic disruption occurred when an ivory lion head (ANE 132697) fragmented following a move to new storage. It was found to have fractured along old cracks which had been consolidated with polyvinyl acetate resin. Soluble salts, gypsum, and halite were present in the cracks and their crystallization at the low relative humidity sustained during the move was considered a contributory factor in the deterioration of the object (Thickett and Bradley 1998). 2.1.4 Control of Relative Humidity in Showcases and Storage AreasFor copper alloy objects susceptible to bronze disease, weeping glass, and ivory, the control of relative humidity in both showcases and storage areas has been achieved by only dehumidifying to maintain a low relative humidity and only humidifying to maintain a higher relative humidity. There has been respectively no counter humidification or dehumidification, and no temperature control other than winter heating. This has meant that for the dehumidified cases the winter low internal relative humidity has been accepted and for humidified cases the summer high relative humidity has been accepted. The relative humidity inside the controlled showcases has drifted accordingly, although the well-sealed showcases in use since 1988 buffer effectively against the peaks and troughs in relative humidity. The fact that no observable damage has occurred to any of the copper alloy or ivory objects in a controlled relative humidity has justified this approach. There have been instances of glass apparently starting to weep in the dehumidified case. Research into this problem is ongoing with the focus on occasional very low relative humidity compared to the target of of 38–42% RH, and high levels of indoor pollutant gases in the dehumidified case. 2.2 COMPLEX MATERIALS2.2.1 Lindow ManWhen Lindow Man, a freeze-dried bog body dated to the Iron Age, was put on exhibition, avoiding the seasonal extremes of relative humidity was thought necessary. Having no idea how the body would react to changes in relative humidity, Museum scientists and conservators were keen to stabilize the environment as much as possible. The body had been immersed in a solution of polyethylene glycol 400 prior to freeze-drying and after treatment was acclimatized to ambient conditions in the Museum (Omar et al. 1989). Because low molecular weight To supply these conditions the showcase was constructed with an integral humidifier (Defensor PH5) and a dehumidifier (Munters M120), both controlled by a Sauter HSC hygrostat. The humidifier was modified with a large funnel that covered the air outlet, connected by large diameter tubing to a hole in the showcase base. The selection of the dehumidifier was based on a comparison of the operation of the condensation and desiccant equipment. Desiccant equipment which had already been used successfully in the Museum was selected. The dehumidifier was connected to the case following the manufacturer's instructions. The showcase was monitored using an electronic RH/temperature probe which showed the relative humidity was tightly controlled in the range 53–57%, but the temperature range was 18–30�C. At 55% RH the moisture envelope surrounding the body was 9–18 g/m3, a much wider range than anticipated. However, physically the body has remained in good condition since it was put on display in 1989. The color post-conservation has apparently lightened and because of this in January 2005 an examination of the body was carried out. This showed the body to be in excellent condition, but some extrusion of deteriorated PEG had occurred. Color monitoring of Lindow Man while on display had indicated that fading occurred during the exhibition period between 1987 and 1997, when the light levels on the body were up to 400 lux. In 1997 Lindow Man was moved to the Late Bronze Age and Celtic Europe Gallery where it is displayed under a maximum light level of 50 lux. Since then the color change has slowed dramatically. 2.2.2 Ethnographic ObjectsThe Museum's Ethnography collection is now stored and displayed at a range of 45–55% RH based on the large number of organic objects. There is no temperature control in the main storage area, but on exhibition, air cooling has been installed to keep the temperature in the galleries below 25�C. Much of the collection is stable but this range of relative humidity promotes the deterioration of some objects. When the deterioration of Maori objects made of New Zealand flax was investigated, the black dye used to color them, an iron/plant polyphenol complex, was found to be the cause (Daniels 1999). The dye was shown to become acidic causing hydrolysis of the cellulose fibers, and the iron in the dye caused oxidation. The research showed that storing the objects at a lower relative humidity would reduce the rate of hydrolysis. However this might affect the dimensional stability of the objects which have acclimatized to the 45–55% range since 1972. The decision was made to keep black dyed New Zealand flax objects in the main storage area. An investigation of the mechanism involved in localized browning of sugar objects from Mexico showed the cause to be Maillard browning, a complex reaction of reducing sugars and protein-containing compounds. The sugar commonly used in cooking and sweetening is sucrose which is not a reducing sugar. However, experiments showed the rate of hydrolysis of sucrose to the reducing sugars, glucose, and fructose, increased with increase in relative humidity. The decoration on the objects was made from icing sugar which contains egg white, the source of the protein. As a result of this research, the objects were moved to a specially created low relative humidity, low temperature storage area (Daniels and Lohneis 1997). 2.2.3 Stone SculptureControl of both relative humidity and temperature which required full air conditioning, was justified for sculptures from the Great Stupa of Amaravati, India. The sculptures were carved in a green-tinged, partially metamorphosed limestone, commonly known as “Palnad marble,” and were deteriorating by powdering and flaking, causing considerable loss of surface detail. Between 1960 and 1992, several campaigns of scientific investigation were undertaken (Bradley and Freestone 1992) identifying five potential causes of deterioration. The cleavage planes created by the folia caused the stone to split readily. The clay minerals in the stone were subject to softening and volume change at high relative humidity, causing surface flaking. Disruption of the surface through powdering was caused by a combination of crystallization and dissolution of soluble chloride along with dissolution and re-precipitation of calcite by reaction with carbon dioxide and water and surface sulfation. This diagnosis suggested that the 2.3 RECENT CHANGES IN CONTROL OF RELATIVE HUMIDITY IN SHOWCASESThe method of control used in showcases has improved in the last eight years. In new gallery developments, a new type of control system is being used instead of humidifiers and dehumidifiers. These controllers, developed by Glausbau Hahn Ltd., utilize a Peltier cell which cools air and hence dehumidifies without the use of refrigerants or drying wheels. It is based on a thermo-electrical effect discovered by Jean Peltier in 1834. The system can control to any range of relative humidity by feeding air into the showcase at the required midpoint at a slow rate. Units of different capacity have been used to control the relative humidity in individual showcases, and from a central unit to groups of showcases in a gallery. The former approach has been used to control the relative humidity in individual small showcases in several galleries and in very large showcases in the Wellcome Trust Gallery where ethnographic objects are displayed. The latter approach has been used to control the relative humidity in all of the showcases in the North America gallery where ethnographic objects are displayed, and in the gallery where objects from Korea are displayed. Passive methods, such as use of conditioned silica gel (Artsorb or Prosorb), are used to control relative humidity in only three showcases in the Museum because of the difficulty of maintaining the in-case conditions when the temperature in the gallery is not controlled and the labor-intensive nature of the installations. Currently there are 103 controlled showcases in the Museum galleries. 3 INDOOR POLLUTANT GASESWhen Werner (1972) published a short paper on the corrosive effects of materials used in showcases, he could not have imagined that 36 years later, scientists in the Museum would still be conducting research into that issue and that there would be an annual conference on indoor air quality. The development of a simple test to screen materials used in the storage and display of objects (Oddy 1973) was followed by research into the gases given off by materials (Blackshaw and Daniels 1978), the conditions under which gases are given off, adsorption of gases by objects, and the formation of uncharacterized mixed salts on objects. The role of the external pollutant gases hydrogen sulfide and carbonyl sulfide in the tarnishing of silver has also been an important area of research in the Museum. 3.1 ACCELERATED CORROSION TESTING OF MATERIALS3.1.1 The Oddy TestSimple corrosion test methodology in which the test material and a metal coupon were heated to 100�C for 3 days was adapted to carry out the first testing of showcase materials. Preliminary studies were conducted to determine the optimum test temperatures using both pure metal and metal alloys. From these tests it was decided that 60�C for 28 days was optimal for testing. At 100�C the test materials degraded beyond recognition, and a lower temperature of 50�C did not cause enough evolution of gases to corrode the test coupons in a reasonable time. Pure silver, copper, and lead coupons were seen to adequately represent the metals of antiquity based on the analytical data available in the Research Laboratory and in the literature at that time. Initially only the fabrics used inside showcases were tested, but it soon became apparent that a much wider range of materials such as paints, woods, adhesives, sealants, and fittings needed to be tested. Two materials emerged as highly problematic for use in showcase construction, wood and wool. The problem of acetic acid being given off by wood and corroding lead had already been published by Scott during the early years of science in the Museum (Scott 1922), and had been known in antiquity (Rackham 1968). In the tests it emerged that wool fabrics always caused silver to tarnish. This was because the sulfide linkages in the protein chains degraded, giving off reduced sulfur gas. Wool was banned from use, but it was not possible to ban the use of wood as at that time all of the showcases in the Museum, including new showcases, were constructed of wood. Since its publication in 1973, the Oddy test has undergone modifications (Oddy 1975, Blackshaw 3.1.2 The 3-in-1 TestCarrying out large numbers of accelerated corrosion tests is time-consuming, and following the publication of a test methodology where one test contained all three metal coupons (Bamberger et al. 1999), a 3-in-1 test was developed for use in the British Museum (Robinet and Thickett 2003). The test methodology devised by Bamberger et al. was not used after finding that the method of deploying the coupons by bending them over the edge of a beaker resulted in contact with water condensation, inducing corrosion that would not normally be observed. The method set out by Robinet utilized a reliable supply of disposable silicone stoppers which fitted the quickfit tubes used for the accelerated corrosion test. The coupons were inserted into slits in the stoppers and the rest of the test set up was as before. For a six-month evaluation period the 3-in1 test and the normal test were run on every material which came in for testing. Currently the 3-in-1 test method is used for the routine testing of materials for use in storage or display of the collection. More complex methods for evaluating materials have been suggested and one has been published (Reedy et al. 1998). This method requires equipment, expertise, and time that are not available in most museum science laboratories. The Oddy test and 3-in-1 test are pass/fail tests which can be carried out by a scientist or conservator who is trained in the test procedure and has a good understanding of laboratory practice. These tests provide a simple way of ensuring that the risk to objects from indoor pollutant gases given off by materials used in storage and display is minimized. 3.2 CARBOXYLIC ACIDS AND ALDEHYDESIndoor pollutant gases are those which are given off by the materials used inside a building. The gases of concern in conservation are those which react on object surfaces to form corrosion. The lower carboxylic acids, up to C4, were identified in emissions from a range of hardwoods, softwoods, and wood products by gas chromatography (GC) headspace analysis and shown to cause corrosion of lead (Blackshaw and Daniels 1978). In practice the main gases of concern are acetic and formic acid, possibly formaldehyde and acetaldehyde, and the reduced sulfur gases, hydrogen sulfide and carbonyl sulfide. 3.2.1 Acetic and Formic AcidsFor manufactured wood products, the most important indoor pollutant is formaldehyde. When composite wood products such as particle boards and Medium Density Fiber board (MDF) were developed, they were quickly taken up by the furniture industry because of their low cost and easy working properties. However, these products give off large quantities of formaldehyde derived from the adhesives used in their manufacture. Formaldehyde was found to affect the health of people and was eventually identified as a carcinogen. Because of this, a passive test for measurement of formaldehyde was developed and was used in several museums including the British Museum; at the time there was no easy quantitative test for acetic and formic acid. Use of the formaldehyde test put undue emphasis on formaldehyde as a main source of corrosion of metals, although laboratory tests in the Museum showed that at concentrations of 0.5 and 5 ppm it did not corrode lead or copper test pieces at 50% RH and temperatures of 15, 25, and 35�C. Slight corrosion of lead test coupons occurred using the same experimental set-up at 100% RH. This suggested that at higher relative humidity, conversion of formaldehyde to formic acid occurs (Thickett et al. 1998). Other researchers suggested that formaldehyde was a more serious problem (Hatchfield and Carpenter 1987). Researchers at the University of East Anglia showed that formaldehyde could be oxidized to formic acid at ambient temperature. However the experimental levels of oxidant were high compared to what normally would be expected in the air (Raychaudhuri and Brimblecombe 2000). Another The wood industry changed formulations and lowor zero-formaldehyde MDF emerged. These products still gave off copious acetic and formic acid and failed the accelerated corrosion test. For more than thirty years in which the accelerated corrosion test has been in use, most of the woods and wood products tested have corroded the lead test coupon. Even ancient wood can corrode lead. An eighthcentury BC lead figurine (1880-12-16-46) formed from tiny lead and ivory squares had a wood core which was found to be the source of the regular corrosion of the lead (Duncan 1986a). In the British Museum the corrosion product identified most frequently on lead objects by X-ray diffraction (XRD) is hydrocerrusite, PbCO3. Pb(OH)2. The corrosion of lead to basic lead carbonate has been described as a two-stage process via an intermediate, lead acetate, which reacts with water and carbon dioxide in the air to form basic lead carbonate. In the last few years, more findings have been made of acetateand formate-containing corrosion products on lead and other metals, and of mixed salts on porous stone, ceramics, and glass. These occur where composite wood products or untested paints are in use. Recent improvements in analysis by ion chromatography (IC) in the Museum have identified the presence of carbonates in salt mixtures which would previously have been identified only as formate and/or acetate. Measurement of the acetic and formic acid levels using the diffusion tube method described by Gibson et al. (1997a) have shown acetic acid to always be present at a higher concentration than formic acid. The prevalence of formatecontaining corrosion and salts suggests that either formic acid is more reactive than acetic acid at some object surfaces, or oxidation of formaldehyde to formic acid is occurring. 3.2.2 Conditions for the Formation of Mixed SaltsFrom a number of incidents of rapid formation of mixed corrosion products and mixed salts on objects, it was suspected that although acetic and formic acid may have adsorbed onto the surface of some objects, the formation of corrosion and efflorescence was dependent on high temperature and high relative humidity, either promoting out-gassing from wood, or promoting the reaction of the adsorbed gases on object surfaces. The formation of acetateand formate-containing corrosion and salts has been investigated on a range of materials including Egyptian copper alloys, limestone, marble, glass, and enamels. On Egyptian bronzes a previously uncharacterized compound, sodium copper carbonate ethanoate (acetate) was identified and characterized (Bradley and Thickett 1999; Thickett and Odhlya 2000). On an Egyptian limestone stela (EA1332) a salt efflorescence containing methanoate (formate), nitrate, and chloride in the ratio 3:2:1 was identified. This is likely to be the corrosion product characterized by Gibson et al. (1997b). During conservation the stela had been poulticed to remove salts and was returned to its oak storage box before fully drying. It is likely that the moisture promoted a reaction between acetic acid from the wood and the soluble salts in the stone forming the mixed salt. The soluble salt profiles through the thickness of this stela and one which had not been treated with water and did not have salt efflorescence present were compared. In addition to the expected chloride and nitrate, acetate and formate ions were present in amounts of 0.01–0.06% w/w throughout both stele (Bradley and Thickett 1999). This shows that gases given off by the oak storage boxes had been adsorbed not only on the surface of the porous limestone but throughout its structure. A white efflorescence had formed on the surface of marble relief (MLA OA 10562) following leakage from a water pipe. This was identified as a calcium acetate formate hydrate (Thickett 1995), a mixed salt which had previously been identified on shells (Tennent and Baird 1985). The relief was mounted in a glass-fronted wood box that was a source of acetic and formic acid. Acetates and formates have been found on the surface of glass and enamels. Many gases readily adsorb onto the surface of glass, and it is highly likely that acetic and formic acids were taken up during long periods of storage in wood cupboards or display in wood showcases. Like many objects around the world, those in the British Museum have traditionally been stored in wood cupboards, resulting in long-term exposure to emissions of acids and aldehydes, which have been adsorbed onto the surface or even throughout the structure of many of the objects. This appears to be a substantial problem, but there are not that many instances of corrosion or salts on objects containing acetate or formate. On investigation, incidents of From the analysis of acetic and formic acid levels in showcases and store cupboards, an empirical relationship between high humidity and temperature and the rate of out-gassing from materials has emerged (Bradley 2003b). In the non-air-conditioned galleries of the Museum there is a seasonal variation in levels of acetic and formic acid and aldehydes, with winter levels considerably lower than summer levels. Since wood is still in use in showcases, albeit wrapped in a barrier film to reduce out-gassing (Thickett 1998), acids and aldehydes are present. However an examination of at-risk objects on display showed that they were not being affected by the gases and lead coupons in showcases did not corrode (Bradley and Thickett 1999). Even very high levels of acetic acid in cupboards used for the storage of Egyptian copper alloy objects did not corrode lead coupons. The relative humidity was at or below 45%. In general, the presence of moisture is needed for mixed corrosion products or mixed salts to form on objects; when conditions are favorable, formation is rapid. Further work is needed to establish if these types of reactions can be eliminated by keeping objects at a low relative humidity. 4 REDUCED SULFUR GASESThe reduced sulfur gases hydrogen sulfide (H2S) and carbonyl sulfide (COS) are important because they form silver sulfide on the surface of silver objects causing them to tarnish, form black sulfides on copper alloy objects, and react with lead pigments causing blackening. There is no need for any material which emits these gases to be used in the storage or display of collections since they can be easily identified during the accelerated corrosion test. However even though only materials which do not tarnish silver are used in the Museum, reduced sulfur gases are present in the external air at concentrations of parts per trillion and are present in the unfiltered air in the Museum. Very low concentrations of these gases can cause silver to tarnish. In the British Museum research into these gases has focused on the tarnishing of silver since this has such an impact on conservation effort and time, with many objects requiring regular cleaning. Silver used to be lacquered to reduce the rate of tarnishing, but removing lacquer was difficult and residues have recently been shown to promote tarnishing (Thickett and Hockey 2003). Another approach was to use silver cleaners which included tarnish inhibitors, usually mercaptan based (Wilthew 1981, 1987). 4.1 TESTING TARNISH PREVENTION PREPARATIONSBetween 1975 and 1987 different types of tarnish-prevention preparation were tested for use in the Museum. The preparations included vapor-phase inhibitors, protective papers and cloth for wrapping or enclosing silver, and absorbent materials. The testing took the form of exposing a cleaned silver coupon and the material under test in a closed container with a source of hydrogen sulfide. Other researchers used either artificially high levels of hydrogen sulfide or carbonyl sulfide (Franey et al. 1985). The effectiveness of the preparations was determined by the number of days taken for tarnish to be visible on the silver coupons. In these tests all of the preparations retarded the formation of tarnish when compared with the time taken for the control coupons to tarnish. However the most effective preparations were 3M silver protector strip, Tarnprufe cloth, four ICI catalysts, of which 75-1 was most effective and Charcoal Cloth (Bradley 1983; 1985; 1989; Duncan 1986b; 1987). As 3M protector strip was designed to be used in smaller closed storage boxes and Tarnprufe cloth, which contained zinc acetate as a sulfide scavenger, was designed to enclose silver, neither was suitable for use in the display of silver objects. ICI catalyst 75-1, a highly porous zinc oxide (wurzite) bonded with cement, designed to remove hydrogen sulfide from North Sea gas, and Charcoal Cloth were the most promising preparations for use in showcases to reduce or remove reduced sulfur gases. In 1993 a more rigorous investigation of silver tarnishing to quantify the efficiency of the tarnish inhibitors and identify the nature of silver tarnish in the Museum began (Lee 1996). The high hydrogen sulfide concentrations used in previous experiments formed a tarnish layer on silver was very much easier to remove than that formed naturally in the Museum galleries and in the laboratory air. As a result it was decided to use concentrations of reduced sulfur gases as near to ambient levels as possible. There was a precedent for conducting corrosion experiments with silver at naturally occurring levels of hydrogen
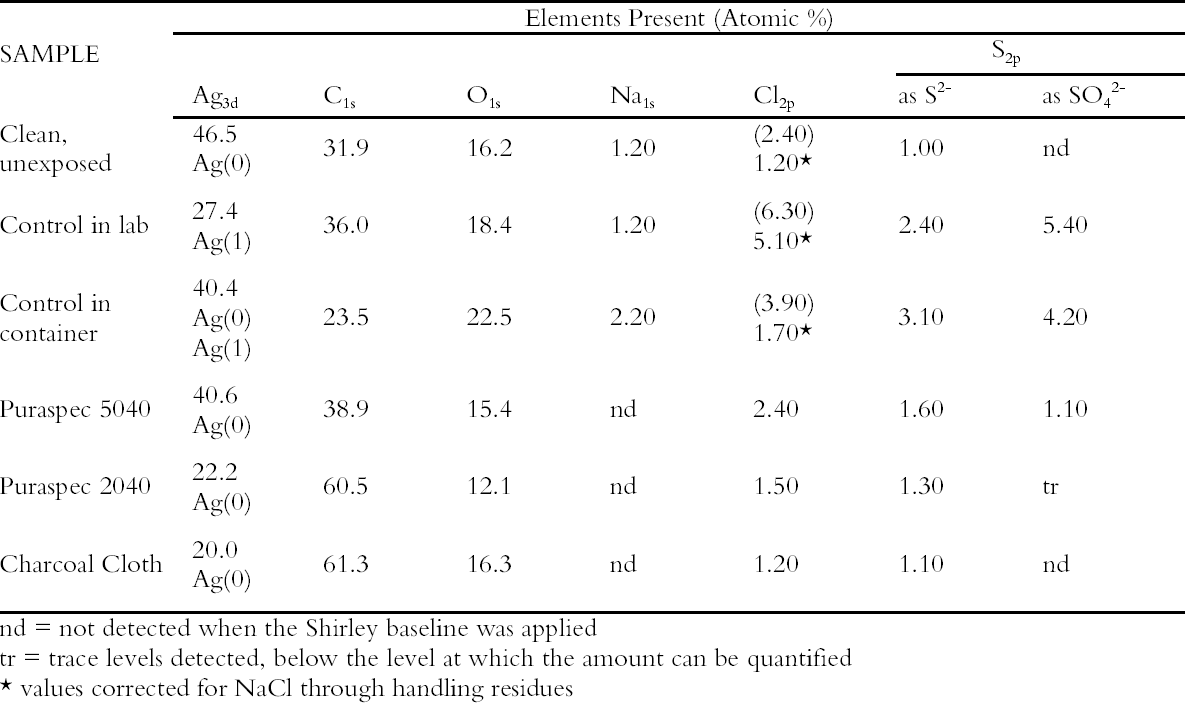
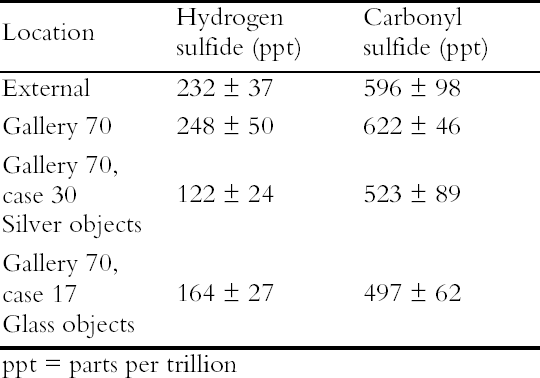
A small aquarium pump was used to pump air through a series of 2L capacity containers. Two holes were drilled into the screw tops of the containers, one for the inlet tubing and one for the air outlet. The containers were connected by rigid Teflon tubing and polythene T-connectors. The arrangement ensured that the pathway to each of the containers was exactly the same length, volume and had the same number of joins and that each container received an equal air flow. Eleven materials were evaluated in this experiment but only the results on the two most effective ICI catalysts, now named Puraspec 5040 and 2040, and on Charcoal Cloth are reported here (Table 1). The materials were placed in individual containers with the cloth cut to cover the base and the Puraspec pellets added to form a monolayer. Analar silver coupons were cleaned by abrasion and degreased in high purity acetone before being pierced and weighed. One coupon was then suspended from the screw top of each container, all at the same height. Controls were set up with a silver coupon suspended in a container alone and one suspended in the area of the apparatus in the laboratory. The experiment was started in July 1994 and completed in April 1995. When the experiment was stopped the control in the laboratory was a dark yellow brown color and that in the container was distinctly yellowed. The silver coupons exposed with the Charcoal Cloth and Puraspec 5040 and 2040 showed no visible change, but at 60x magnification, black spots could be seen. 4.1.1 Analysis of Silver Test CouponsPrior to analysis the coupons were individually stored in bags made from a film with low permeability to gases along with sachets of Ageless oxygen scavenger to prevent further change. The coupons were analyzed by X-ray photoelectron spectroscopy (XPS) with a Kratos XSAM series 800 using Mg and Al Kα radiation at 300 W using 7.5mm slits and 80/40 eV pass energies. The results in Table 1 (Johnson Matthey Technology Centre 1995) show that the silver tarnish contained oxides, chlorides, and sulfates in addition to the expected sulfide. On the control coupons, sulfide was present at lower concentrations than oxide and sulfate. Despite efforts to minimize handling of the samples the presence of sodium suggested handling contamination on some of the coupons and an 4.2 REDUCED SULFUR GAS MONITORINGAs part of a broader pollutant gas monitoring campaign in the Museum, hydrogen sulfide and carbonyl sulfide levels were monitored using diffusion tubes supplied by S. Watts of Oxford Brookes University, Oxford, UK. The tubes and silver coupons were deployed in Gallery 70, Rome City and Empire, in the gallery, in two showcases containing silver objects, in a control case containing only glass, and outside of the Museum building for 28 days. The showcases were different sizes but were constructed to the same specification, and the case inserts, dressing fabrics and label materials had been tested to ensure that they did not out-gas reduced sulfide gases. The analysis of reduced sulfur gases was interesting, as carbonyl sulfide was present in greater concentration than hydrogen sulfide at all of the locations monitored. The levels inside showcases were almost as high as the levels in the gallery and externally (Table 2). Only the coupon exposed externally was visibly tarnished, particularly at the edges. Taking the measurement
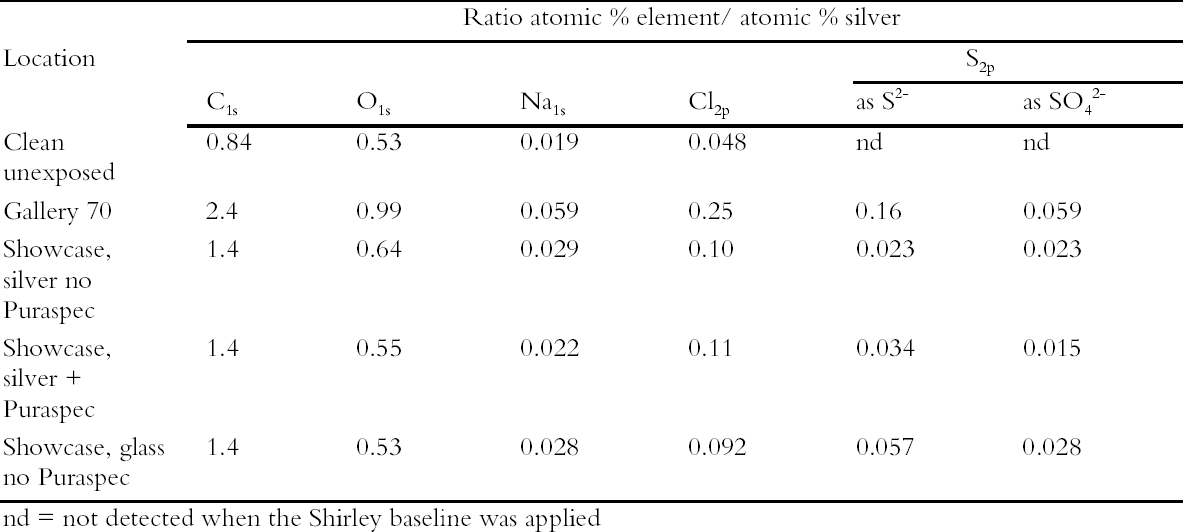
These coupons were analyzed by XPS and Static Secondary Ion Mass Spectrometry (SSIMS). The SSIMS analysis was carried out using a Millbrook Chemical Microscope in large area (2.25 mm2), static mode for both negative and positive ions. The primary beam is provided by a raster scanned gallium liquid metal ion gun with low energy optics for secondary ion extraction into a 300 Da quadropole mass spectrometer. Although both methods are surface analysis techniques the information they provided is different. In XPS the elements present are separated according to their binding energy (eV) and hence sulfur as sulfide and as sulfate are discriminated. The analysis is quantified as atomic concentration percent. SSIMS is a non-quantitative technique and the output is a mass spectrum detecting fragments as well as ions. Sodium, chlorine, oxygen, zinc, sulfur as sulfide and as sulfate, silicon, and carbon were detected on the surface of the silver coupons. 4.3 THE NATURE OF SILVER TARNISHIn this program of monitoring, the nature of silver tarnish in the British Museum was confirmed as a mixture of oxide, chloride, sulfate, and sulfide. Carbon was again a major contaminant on the surface of the coupons with fragments of up to C6 in chain length present as determined by SSIMS. In this work, handling of the coupons was dramatically reduced by mounting them on aluminum stubs for presentation in the SSIMS equipment and storage in aluminum containers prior to exposure and analysis. Hence carbon species in the atmosphere were indicated as the primary source of this contamination (Hallett et al. 2003). Carbonyl sulfide was shown to be the predominant reduced sulfur gas in the air and not hydrogen sulfide; hence, the strategy of basing a tarnish prevention system on a material designed to remove hydrogen sulfide was flawed. 4.4 GALLERY TRIALSMeanwhile the evaluation of the passive deployment of Puraspec 5040 was continued in gallery trials. The pellets were deployed inside a large Lshaped showcase displaying silver objects and were visible. Silver coupons were put into this case and
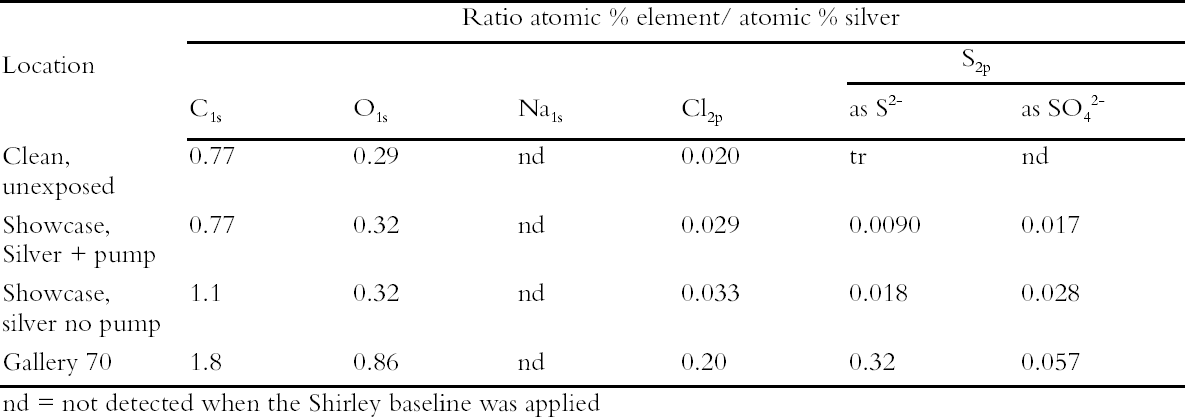
The coupons in the control locations tarnished after 42 days. All of the coupons were then analyzed by XPS. The results are presented in Table 3 as ratio atomic percent. There was more sulfur present on the silver coupon exposed in the showcase containing glass objects than that containing silver objects and no Puraspec. Hence the trial showed that silver objects are likely to tarnish at a slower rate in showcases containing other silver objects. This is presumably because the silver objects act as a sink for reduced sulfur and other gases. The Puraspec 5040 in passive mode did not reduce the rate of tarnishing. The results of this experiment were later found to have been affected by the high air exchange rate of the complex L-shaped showcase, more than 8 air changes per day. A trial of a positive pressure system was undertaken replacing Puraspec 5040 with Puraspec 2030 which was suggested by ICI Katalco. This product is also formed from zinc oxide and contains aluminum oxide which oxidizes carbonyl sulfide to hydrogen sulfide which is in turn absorbed by the zinc oxide, and a copper compound which acts as an indicator, turning from green to black when it takes up sulfides. A simple filter bed was prepared in a tubular container using Puraspec 2030 and activated carbon cloth. It was decided to incorporate the cloth since it absorbs a broad range of pollutant gases and pumping air into a case could lead to a build up of pollutants at the surface of the objects. The filter bed was connected through the base of a showcase displaying only silver by plastic tubing and a small pump. Silver coupons were deployed to monitor the rate of tarnish formation and these were visually tarnished after 60 days. Again they were analyzed by XPS which showed that the pump had been effective at reducing sulfide formation (Table 4). On the basis of this research, current thought is to use positive pressure systems in all showcases where silver objects are displayed. 5 CONCLUSIONSGreater understanding of the interaction of the museum environment with the collection has been achieved through research by the scientists in the British Museum. Increase in knowledge has been incremental, with improvements in scientific technology providing the capability to extend the work into levels of detail unimaginable in 1970. So that actual object needs are met in a cost-effective way, Museum scientists conducted object-based research designed to inform the preventive conservation strategy (Bradley 1996), rather than advocating wholesale implementation of conditions taken from the literature. Full air-conditioning of the building is not appropriate given the historical significance of the building itself, the range of materials and their levels of deterioration; and the mixed object displays which
The research has been utilized both in the British Museum and in the wider museum community informing showcase and storage unit design. It has prompted manufacturers to move away from wood to metal storage units, and glass and metal showcases, and to develop in-case conditioning systems which are superior to those based on humidifiers and dehumidifiers which we used for 25 years. Design of new galleries and storage facilities incorporates conservation requirements such as air cooling and filtration which are justified by the research. Most importantly, in new galleries and new storage areas, the objects are displayed and stored in safe conditions that are a vast improvement over those which existed in 1970 when this research was begun. ACKNOWLEDGEMENTSI would like to thank all the members of the Conservation Science team who have worked over the years to develop our understanding of deterioration and the Museum environment. In particular Lorna Lee (n�e Green) and David Thickett have made a substantial contribution to this research. I would like to acknowledge the help of the conservators, curators, museum assistants, designers, particularly Geoff Pickup and buildings staff who point out incidents of objects changing, help with measurements in galleries and stores, and implement solutions. I would also like to thank Sheridan Bowman for reading the manuscript and suggesting improvements. REFERENCESBamberger, J. A., E. G.Howe, and G.Wheeler. 1999. A variant Oddy test procedure for evaluating materials used in storage and display cases. Studies in Conservation44:86–90. Bimson, M.2004. Personal communication. Retired scientist, Research Laboratory, The British Museum, London. Blackshaw, S. M., and V. D.Daniels. 1978. Selecting safe materials for use in the display and storage of antiquities. ICOM Committee for Conservation preprints. 5th Triennial Meeting, Zagreb. 78/23/2/1-9. Blackshaw, S. M., and V. D.Daniels. 1979. The testing of materials for use in storage and display in museums. The Conservator3:16–19. Boyd, A.1971. Unpublished report and meeting notes, British Museum Research Laboratory file 2874. London. BradleyS. M.1983. Examination of Tarnprufe impregnated cloth for protection of silver from tarnishing, Conservation News22: 11. Bradley, S. M.1985. Testing anti-tarnish preparations. In Corrosion inhibitors in conservation, UKIC Occasional Papers 4, ed. S.Keene, London: UKIC. 2123.
Bradley, S. M.1989. Hydrogen sulfide scavengers for the prevention of silver tarnishing. In Environmental monitoring and control, Dundee: SSCR and the Museums Association. 6567. Bradley, S. M.1996. Development of an environmental policy for the British Museum. ICOM Committee for Conservation preprints. 11th Triennial Meeting, Edinburgh. London: James and James Science Publishers Ltd.1:8–13. Bradley, S.2003a. The scientific investigation and preventive conservation of the Amaravati sculptures in the British Museum. In Conservation of stone, ed. V.Jeyaraj, Chennai: The Government Museum Chennai. 189–196. Bradley, S.2003b. Studies on the susceptibility of objects to pollutant gases. Abstract. Indoor Air Quality in Museums and Historic Properties. IAQ 2003: 5th meeting of the Indoor Air Pollution Working Group, Norwich, UK. http://iaq.dk/iap/iaq2003/2003_contents.htm (accessed 3/03/06). Bradley, S. M., and I.Freestone. 1992. A note on the chemistry and petrology of the Amaravati stone, its susceptibility to deterioration and the strategy for its preservation. In R. Knox, Catalogue of the Amaravati sculptures. London: British Museum Press. 230–231. Bradley, S., and D.Thickett. 1999. The pollution problem in perspective. ICOM Committee for Conservation preprints. 12th Triennial Meeting, Lyon. London: James and James Science Publishers Ltd.2:8–13. Daniels, V.1999. Factors affecting the deterioration of the cellulosic fibres in black dyed New Zealand flax. Studies in Conservation44:73–85. Daniels, V., and G.Lohneis. 1997. Deterioration of sugar artefacts. Studies in Conservation42:17–26. Daniels, V. D., and S.Ward. 1982. A rapid test for the detection of substances which will tarnish silver. Studies in Conservation27:58–60. Duncan, S.1986a. An investigation into the mechanism(s) of active corrosion on the Toprakale figure (1880-12-16-46). Conservation Research Report V20, The British Museum, London. Duncan, S.1986b. Protection of silver against sulfide tarnishing. Conservation Research Report V9, The British Museum, London. Duncan, S.1987. Evaluation of four different hydrogen sulfide scavengers. Conservation Research Report V30, The British Museum, London. Franey, T. P., G. W.Kammlott, and T. E.Graedel. 1985. The corrosion of silver by atmospheric sulfurous gases. Corrosion Science25, 2:133–143. Gibson, L. T., B. G.Cooksey, D.Littlejohn, and N. H.Tennent. 1997a. A diffusion tube sampler for the determination of acetic acid and formic acid vapours in museum cabinets. Analytica Chimica Acta341:11–19. Gibson, L. T., B. G.Cooksey, D.Littlejohn, and N. H.Tennent. 1997b. Characterisation of an unusual crystalline efflorescence on an Egyptian limestone relief. Analytica Chimica Acta337:51–164. Green, L. R., and D.Thickett. 1993. Interlaboratory comparison of the Oddy test. In Conservation Science in the UK, ed. N.Tennent, London: James and James Science Publishers Ltd.111–116. Green, L. R., and D.Thickett. 1995. Testing materials for the storage and display of artefacts—a revised methodology. Studies in Conservation40:145–152. Hallett, K., D.Thickett, D.McPhail, and R.Chater. 2003. Application of SIMS to silver tarnish at the British Museum. Applied Surface Science 2003-2004: 789–792. Hatchfield, P., and J.Carpenter. 1987. Formaldehyde: How great is the danger? Cambridge, MA: Harvard University Arts Museum. Johnson Matthey Technology Centre. 1995. Report on XPS analysis carried out by J. A. Busby at Johnson Matthey Technology Centre, Blount's Court, Sonning Common, Reading, UK. Lee, L. R.1996. Investigation of materials to prevent the tarnishing of silver. Conservation Research Report 1996/1, The British Museum, London. Lee, L. R., and D.Thickett. 1996. Selection of materials for the storage or display of museum objects. British Museum Occasional Paper 111. London: The British Museum.
Newey, N.1987. 17 years of dehumidified showcases in the British Museum. ICOM Committee for Conservation preprints. 8th Triennial Meeting, Sydney Oddy, W. A.1973. An unsuspected danger in display. Museums Journal73:27–28. Oddy, W. A.1975. The corrosion of metals on display. In Conservation in archaeology and the applied arts, ed. N. S.Brommelle and P.Smith. London: International Institute for Conservation of Historic and Artistic Works. 235–237. Omar, S., M.McCord, and V.Daniels. 1989. The conservation of bog bodies by freeze-drying. Studies in Conservation34:101–109. Organ, R.1963. The examination and treatment of bronze antiquities. In Recent advances in conservation, ed. G.Thomson. London: Butterworths. 104–110. Organ, R., and M.Bimson. 1957. The safe storage of unstable glass. The Museums Journal56:265–272. Plenderleith, H. J.1956. The conservation of antiquities and works of art. London: Oxford University Press. 1–15. Pope, D., H. R.Gibbens, and R. L.Moss. 1968. The tarnishing of silver at naturally occurring H2S and SO2 levels. Corrosion Science8:883–887. Rackham, H. (Translator).1968. Pliny's Natural History. London: W. Heinemann, XXXIV, LV. 253. Raychaudhuri, M. R., and P.Brimblecombe. 2000. Formaldehyde oxidation and lead corrosion. Studies in Conservation45:226–232. Reedy, C. L., R. A.Corbett, and M.Burke. 1998. Electrochemical tests as alternatives to current methods for assessing effects of exhibition materials on metal artifacts. Studies in Conservation43:183–196. Robinet, L., and D.Thickett. 2003. A new methodology for accelerated corrosion testing. Studies in Conservation48:263–268. Schmidt, S.1992. The formation of sodium formate on glass surfaces: examination of historical objects. Berliner Beitr�ge Arch�ometrie11:137–183. Scott, A.1922. The restoration and preservation of objects at the British Museum. Journal of the Royal Society of ArtsLXX(3): 327–338. Tennent, N. H., and T.Baird. 1985. The deterioration of Mollusca collections: identification of shell efflorescence. Studies in Conservation30:73–85. Thickett, D.1995. The analysis of salt efflorescence and investigation of the cause of staining of a marble Carpenter relief, MLA OA 10562. Conservation Research Report CA1995/41, The British Museum, London. Thickett, D.1998. Sealing of MDF to prevent corrosive emissions. The Conservator22:49–56. Thickett, D., and S.Bradley. 1998. Analysis of damaged ivory lion's head WAA 132697. Conservation Research Group Report 1998/6, The British Museum, London. Thickett, D., S.Bradley, and L.Lee. 1998. Assessment of the risk to metal artefacts posed by volatile carbonyl pollutants. In Metal 98, eds. W.Mourey and L.Robbiola. London: James and James Science Publishers Ltd.260–264. Thickett, D., and M.Hockey. 2003. The effects of conservation treatments on the subsequent tarnishing of silver. In Conservation science 2002, eds. J.Townsend, K.Eremin, and A.Adriaens. London: Archetype Publications Ltd.155–161. Thickett, D., and L. R.Lee. 2004. Selection of materials for the storage or display of museum objects. British Museum Occasional Paper 111. Revised edition. London: British Museum. Thickett, D., and M.Odhlya. 2000. Note on the identification of an unusual pale blue corrosion product from Egyptian copper alloy artefacts. Studies in Conservation45:63–67. Thomson, G.1978. The museum environment. Oxford: Butterworth-Heinemann.
Toishi, K., and H.Washizuka. 1987. Characteristics of Japanese art that condition its care. Tokyo: The Japanese Association of Museums. Werner, A. E. A.1958. The conservation of glass. In Congr�s international d'etude du verre, ed. J.Philippe. Liege: Journees Internationales du Verre. Werner, A. E. A.1972. Conservation and display: environmental control. Museums Journal72(2): 58–60. Wilthew, P.1981. The effect of cleaning treatments on the long term tarnishing of silver. Conservation Research report III 13, The British Museum, London. Wilthew, P.1987. Evaluation of four different hydrogen sulphide scavengers. Conservation Research Report V 30, The British Museum, London. Zang, J., D.Thickett, and L. R.Green. 1994. Two tests for the detection of volatile organic acids and formaldehyde. Studies in Conservation33:47–53. AUTHOR INFORMATIONSUSAN BRADLEY, Head of Conservation Science and Analytical Chemistry in The British Museum Department of Conservation, Documentation and Science, has a degree in chemistry from the University of London. In 1972 she joined the Museum to work on conservation problems, researching conservation methods for waterlogged wood and leather, the deterioration and conservation of stone, metals, ceramics, and glass, the storage of collections, and the museum environment. In 1988 she became Head of Conservation Research Group, leading a small team of scientists on object-centred research. Currently her main interests are the deterioration of glass and enamel and the museum environment. Address: Department of Conservation, Documentation and Science, The British Museum, Great Russell St., London WC1B 3DG, UK
 Section Index Section Index |