FUNORI: OVERVIEW OF A 300-YEAR-OLD CONSOLIDANTJOSEPH R. SWIDER, & MARTHA SMITH
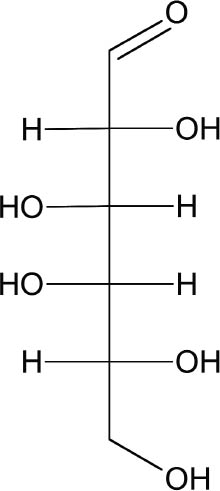
2 SCIENTIFIC SURVEYProcessed seaweeds easily dissolve in water, and their swelling and colloidal nature make them commercially useful. Seaweed gums are natural carbohydrate polymers, composed of repeating units of saccharides (sugars). Traditional seaweed taxonomy is based on qualities such as color, structural properties, and odor (Stancioff 1980). Red seaweeds of the family Rhodophyta are loosely defined by their color, and subsequent classification has been based on chemical structure and origin. Rhodophyta are grouped into three types: agars, carrageenans, and porphyrans. Funori is considered a subset of agars; however, because its chemical and structural qualities are close to those of carrageenans, our study will focus on both agars and carrageenans. Porphyrans, not used commercially or in conservation, will not be discussed. Agar comes chiefly from the two species Gelidium and Gracilaria, and its largest commercial producer is Japan, where it has been cultivated since the 17th century (Percival and McDowell 1967). The many commercial advantages of agar include its ability to gel at a low concentration (as low as 1%) and at room temperature, and its resistance to microbiological agents and most simple enzymes (Araki and Arai 1955; Araki 1965; Meer 1980). Most carrageenans belong to the Chondrus and Gigartina species found on the northern coasts of most countries in the world. These two types of Rhodophyta are based on variations of the monosaccharide galactose (fig. 2) and referred to as the galactans of the Rhodophyta. Polysaccharide chains in agars and carrageenans are based on two repeating forms of galactose, �-D-galactose and an anhydride form, 3,6-anhydro-α-L or D-galactose (fig. 3) that form a double helix. Traditionally,
Recent scientific studies have separated funori into structurally homogeneous fractions to better understand its structure and taxonomy. The fractions indicate the amount (usually in percent of the entire carbohydrate polymer) of a common unit or homogeneous monomer in the seaweed, i.e., a fraction of 3,6-anhydro-α-L-galactose. In general, the most variation among species is in the amount of anhydride (Hirase and Watanabe 1971; Takano et al. 1999). Like funori in its native state, none of the fractions gel. The same methods of fractionation on carrageenan revealed structural similarities to funori (Takano et al. 1998), notably that funori fractions were found in carrageenan polysaccharides. Recent studies point to the fact that carrageenan “backbones,” or the 3,6-anhydro-α-D-galactose sulfated components, were also found in funorans (Takano et al. 1999).
This similarity should be kept in mind when comparing the physical properties of the agars and funori with the carrageenans, in particular for solubility and gelling. The carbohydrate chains of red seaweeds line up together in a “hollow helix” structure (Lobban and Wynne 1981), with side groups of each unit influencing gelling ability. Agar, empty of sulfate groups, will gel at low temperatures and at low concentrations because the helical structures are able to pack tightly due to lack of electrostatic and steric hindrance (Duckworth et al. 1971). Carrageenans and funorans contain sulfate groups that cause the helices to repel one another; although viscous, they do not readily gel. Unlike funori, carrageenan has been commercially available for decades and is routinely separated and sold as fractions under Greek prefixes (i.e., K-i,-6-), each for a different application (i.e., food additives, marbling). Carrageenan is sold commercially as a powder with a cationic salt added (usually the infraction) to remove the sulfate repulsion and form a gel with the cation as a counterion for the sulfate groups. Although not previously reported in the literature, our laboratory has shown that funori will also gel with salts (i.e., potassium chloride) in approximately the same concentration as typical commercial carrageenan. In addition, funori was heated in both tap and deionized water. Neither heat nor water type had an effect on the pH. Scientific studies that have attempted to sulfate polysaccharides such as agarose, starch, and porphyrans having no sulfate groups showed that C-6 is the preferential site for sulfate substitution (Takano et al. 1996). Consequently, this is the site normally sulfated on the funorans but not on the carrageenans, possibly because the C-6 placement is more sterically favored in funori. Although this specificity could imply greater reactivity for the funorans on treated materials in terms of sulfuric acid production, these seaweeds have never been shown to be acidic and this addition may be beneficial as protection. Because of this unique structure and composition, funorans have shown promise as an antitumor agent and as a mouthwash, owing to their apparent antimicrobiological activity (Ren et al. 1994; Ren et al. 1995; Saeki et al. 1996). That there is no record in the literature or in our laboratories of microbiological attack on funori-treated artifacts somewhat supports this antimicrobiological property, but further scientific studies are warranted. When these seaweeds (mostly carrageenans) are used as food additives, a polysaccharide with greater anhydride character is used for gelling applications whereas one with less anhydride and more sulfate character is employed for a viscous solution (Percival 1970). It could be concluded from the above information that funori typically having a single sulfate constituent and anhydride per unit may strike a balance and create an optimal consolidant. |