FUNORI: OVERVIEW OF A 300-YEAR-OLD CONSOLIDANTJOSEPH R. SWIDER, & MARTHA SMITH
ABSTRACT—Funori, a product from seaweed found mostly in Japan, has been used as an adhesive and a consolidant for centuries. Many conservators are familiar with funori but may be unaware of its chemistry and reliable methods for its preparation. Funori has been used successfully for decades at the Freer Gallery of Art and Arthur M. Sackler Gallery's Department of Conservation and Scientific Research in the paper, East Asian paintings, and object conservation laboratories. This article is a review of the conservation and scientific literature to give conservators insight into the chemistry of funori and related seaweeds and to propose methods for its preparation. TITRE—Le funori: un consolidant utilis� depuis 300 ans. R�SUM�—Le funori, un produit d�riv� des algues que l'on retrouve surtout au Japon, a �t� utilis� comme adh�sif et consolidant depuis des si�cles. De nombreux restaurateurs utilisent le funori, mais connaissent peu ses propri�t�s chimiques ainsi que des proc�d�s fiables pour sa pr�paration. Le funori a �t� utilis� avec succ�s depuis plusieurs d�cennies aux laboratoires de restauration des papiers, des peintures asiatiques et des objets de la Freer Gallery of Art/ Arthur M. Sackler Gallery (mus�e d'art Freer/galerie Arthur M. Sackler). Cet article pr�sente une revue des publications dans les domaines de la science et de la restauration, dans le but de fournir aux restaurateurs quelques r�ponses sur les propri�t�s chimiques du funori et autres algues, et afin de proposer des m�thodes ad�quates pour sa pr�paration. TITULO—Funori: vision general de un consolidante de 300 a�os. RESUMEN—El funori, un producto de algas marinas que se encuentran mas que todo en el Jap�n, ha sido utilizado durante siglos como adhesivo y consolidante. Muchos conservadores tienen familiaridad con el funori pero pueden no conocer su composici�n qu�mica y los metodos confiables para su preparacion. El funori ha sido utilizado exitosamente por d�cadas en el Departamento de conservaci�n e investigaci�n cient�fica de la Freer Gallery of Art/Arthur M. Sackler Gallery (Galeria de arte Freer/Galeria Arthur M. Sackler), en los laboratorios de conservaci�n de papel, de pinturas del este asi�tico y de objetos. Este art�culo es una rese�a de la literatura de conservaci�n y cient�fica con el objeto de proporcionar a los conservadores una visi�n general sobre la qu�mica del funori y otras algas marinas semejantes y para proponer metodos para su preparaci�n. T�TULO—Funoroi: Observa��es Gerais dos 300 Anos Do Consolidante. RESUMO—Funori, um produto de algas encontradas basicamente no Jap�o, tem sido utilizado h� s�culos, como adesivo e consolidante. Muitos conservadores est�o familiarizados com o funori, mas talvez desconhe�am sua qu�mica e m�todos confi�veis para seu preparo. Funori tem sido utilizado com sucesso por d�cadas na Freer Gallery of Art and Arthur M. Sackler Gallery's Department of Conservation and Scientific Research (Galeria de Arte Freer, Departamento de Conserva��o e Pesquisa Cient�fica Da Galeria M. Sackler) nos laborat�rios de conserva��o de papel, de pinturas do Leste Asi�tico e de objetos. Este artigo � uma revis�o da literatura cient�fica e de conserva��o para dar aos conservadores informa��es sobre a qu�mica do funori e algas relacionadas e tambem propor m�todos para seu preparo. 1 INTRODUCTIONOver the last century conservation has seen an abundance of synthetic consolidants for adhering flaking paint and mending small fractures. The seaweed product funori, first used more than 300 years ago, is still produced today and can rival many other traditional and synthetic consolidants for its ease of use in a nontoxic form. Although funori has been employed for many years, references to its use and study are scarce. This article surveys the conservation and scientific literature on the use and chemical properties of funori. Preparation guidelines are provided for those who use funori or desire to experiment with it in their laboratories.
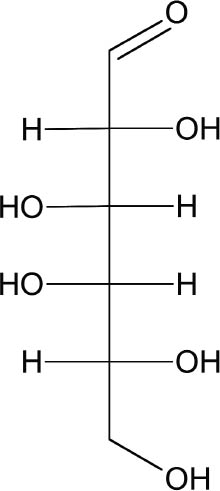
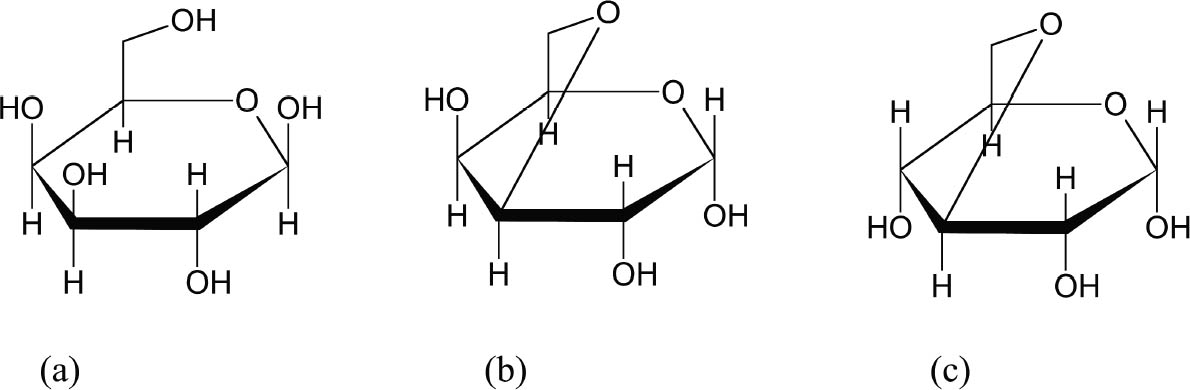
Funori is extracted from any of three species of red seaweed found mostly on the coasts of Japan: Gloiopeltis tenax (mafunori), Gloiopeltis complanata (hana-funori), or Gloiopeltis furcata (fukuro-funori), collectively referred to as the funorans. It is first recorded in Japan in 1673 as a size for textiles and papers and even as a cement for tiles and building materials (Chapman 1980). The seaweed is cultivated on rocks in Japan and is produced year-round. Processing funori is minimal: it is washed and soaked to remove debris and salts (fig. 1). Many modern processes use a bleaching agent such as potassium 2 SCIENTIFIC SURVEYProcessed seaweeds easily dissolve in water, and their swelling and colloidal nature make them commercially useful. Seaweed gums are natural carbohydrate polymers, composed of repeating units of saccharides (sugars). Traditional seaweed taxonomy is based on qualities such as color, structural properties, and odor (Stancioff 1980). Red seaweeds of the family Rhodophyta are loosely defined by their color, and subsequent classification has been based on chemical structure and origin. Rhodophyta are grouped into three types: agars, carrageenans, and porphyrans. Funori is considered a subset of agars; however, because its chemical and structural qualities are close to those of carrageenans, our study will focus on both agars and carrageenans. Porphyrans, not used commercially or in conservation, will not be discussed. Agar comes chiefly from the two species Gelidium and Gracilaria, and its largest commercial producer is Japan, where it has been cultivated since the 17th century (Percival and McDowell 1967). The many commercial advantages of agar include its ability to gel at a low concentration (as low as 1%) and at room temperature, and its resistance to microbiological agents and most simple enzymes (Araki and Arai 1955; Araki 1965; Meer 1980). Most carrageenans belong to the Chondrus and Gigartina species found on the northern coasts of most countries in the world. These two types of Rhodophyta are based on variations of the monosaccharide galactose (fig. 2) and referred to as the galactans of the Rhodophyta. Polysaccharide chains in agars and carrageenans are based on two repeating forms of galactose, �-D-galactose and an anhydride form, 3,6-anhydro-α-L or D-galactose (fig. 3) that form a double helix. Traditionally,
Recent scientific studies have separated funori into structurally homogeneous fractions to better understand its structure and taxonomy. The fractions indicate the amount (usually in percent of the entire carbohydrate polymer) of a common unit or homogeneous monomer in the seaweed, i.e., a fraction of 3,6-anhydro-α-L-galactose. In general, the most variation among species is in the amount of anhydride (Hirase and Watanabe 1971; Takano et al. 1999). Like funori in its native state, none of the fractions gel. The same methods of fractionation on carrageenan revealed structural similarities to funori (Takano et al. 1998), notably that funori fractions were found in carrageenan polysaccharides. Recent studies point to the fact that carrageenan “backbones,” or the 3,6-anhydro-α-D-galactose sulfated components, were also found in funorans (Takano et al. 1999).
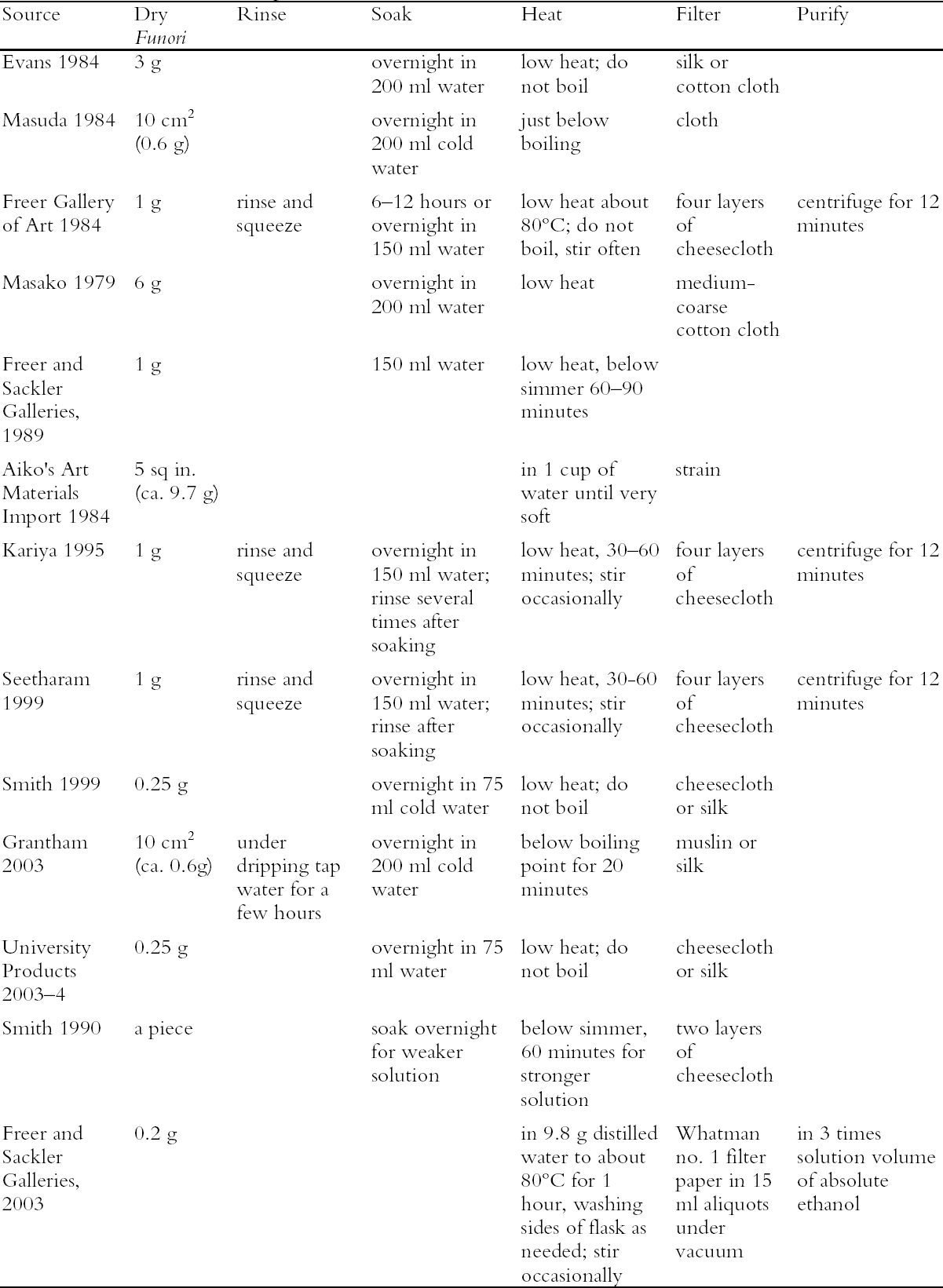
This similarity should be kept in mind when comparing the physical properties of the agars and funori with the carrageenans, in particular for solubility and gelling. The carbohydrate chains of red seaweeds line up together in a “hollow helix” structure (Lobban and Wynne 1981), with side groups of each unit influencing gelling ability. Agar, empty of sulfate groups, will gel at low temperatures and at low concentrations because the helical structures are able to pack tightly due to lack of electrostatic and steric hindrance (Duckworth et al. 1971). Carrageenans and funorans contain sulfate groups that cause the helices to repel one another; although viscous, they do not readily gel. Unlike funori, carrageenan has been commercially available for decades and is routinely separated and sold as fractions under Greek prefixes (i.e., K-i,-6-), each for a different application (i.e., food additives, marbling). Carrageenan is sold commercially as a powder with a cationic salt added (usually the infraction) to remove the sulfate repulsion and form a gel with the cation as a counterion for the sulfate groups. Although not previously reported in the literature, our laboratory has shown that funori will also gel with salts (i.e., potassium chloride) in approximately the same concentration as typical commercial carrageenan. In addition, funori was heated in both tap and deionized water. Neither heat nor water type had an effect on the pH. Scientific studies that have attempted to sulfate polysaccharides such as agarose, starch, and porphyrans having no sulfate groups showed that C-6 is the preferential site for sulfate substitution (Takano et al. 1996). Consequently, this is the site normally sulfated on the funorans but not on the carrageenans, possibly because the C-6 placement is more sterically favored in funori. Although this specificity could imply greater reactivity for the funorans on treated materials in terms of sulfuric acid production, these seaweeds have never been shown to be acidic and this addition may be beneficial as protection. Because of this unique structure and composition, funorans have shown promise as an antitumor agent and as a mouthwash, owing to their apparent antimicrobiological activity (Ren et al. 1994; Ren et al. 1995; Saeki et al. 1996). That there is no record in the literature or in our laboratories of microbiological attack on funori-treated artifacts somewhat supports this antimicrobiological property, but further scientific studies are warranted. When these seaweeds (mostly carrageenans) are used as food additives, a polysaccharide with greater anhydride character is used for gelling applications whereas one with less anhydride and more sulfate character is employed for a viscous solution (Percival 1970). It could be concluded from the above information that funori typically having a single sulfate constituent and anhydride per unit may strike a balance and create an optimal consolidant. 3 LABORATORY STUDIES ON FUNORI PREPARATIONA survey of the conservation literature reveals a number of funori preparations, which are compiled in table 1 together with preparations used at the Freer and Sackler Galleries. In the scientific studies previously mentioned, raw funori is usually washed and given no further alkali treatment, as is typically done with carrageenans. Some methods only add funorans to boiling water (Takano et al. 1999); others soak and macerate it in a blender before adding it to boiling water (Takano et al. 1995; Takano et al. 1998). Many references in the conservation literature fail to give specific methods of preparation (Higuchi 1979; Oka 1988). Some references indicate a solution between 1% and 5% (Masuda 1984; Keyes 1986; Horie 1990; Kariya 1995) but do not provide dilution factors or a purchase origin. The fact that funori is seldom treated before being sold to conservation studios casts doubts on its purity for use on cultural artifacts. Agar has been purified and separated for decades by a simple freeze-thaw method (Grabar 1959; Sewer 1983). As it thaws, agar remains a gel at room temperature while the water in it melts and is removed, presumably containing excess salts. Our freeze-thaw tests on funori showed that like most carrageenans, it cannot be purified by this method. We attempted to purify funori to produce a solution that was free of pulp and obvious extraneous material. A stock solution of 2% dry funori in distilled water (weight per weight) was heated at about 90�C for one hour and stirred occasionally. We used vacuum filtration with Whatman no. 1 filter paper, adding approximately 15 ml at a time because the filter quickly became clogged. The filtered product was clear of most fibrous pulp and other debris, and tinted slightly amber. It was almost clear when painted out on glass. It is a common practice in seaweed studies to precipitate a seaweed solution with ethanol, and we tested this practice as a final step. When approximately three times the filtrate volume
Our laboratory purchased commercial funori and prepared it in a variety of ways to see if there was one standard “best” method. We divided the process into six stages: rinse, soak, heat, extract, dry, and reconstitute.
Preparing funori is a flexible procedure, and overall our attempts successfully produced a material that was free of debris and suitable for treating artifacts. 4 USES OF FUNORI IN CONSERVATIONThe same flexibility applies to the ways in which funori is used, normally for applications that require a low-strength consolidant, usually on paper. The advantages of funori as a consolidant are many: it has good penetrating ability and wets surfaces easily, dries matte, can be diluted with water, and is nontoxic. Uses for funori in conservation include sizing paper and textiles; consolidating flaking and powdering paint; repairing and facing East Asian painting and works on paper (Keyes 1986; Smith 1990); repairing silver and gold leaf and mica (Higuchi 1979; Kariya 1995); backing paintings when mixed with animal glue (Oka 1988); and repairing the structure of wooden objects (Tsujimoto 1979). In Japan it has been used to adhere tiles and paper to walls and as a cement in plaster wall construction and in porcelain decoration. Most authors note the advantages of funori as a low-strength consolidant with low surface tension and low gloss (Higuchi 1979; Michel et al. 2002). One study has examined its effects over time with artificial aging (Michel et al. 2002). This study examined To confirm funori's properties, we have examined previously treated artwork in the Freer Gallery of Art, where funori is used in paper and East Asian painting conservation regularly. A concentrated solution of funori is made up and thinned to suit the treatment at hand. Often the funori is applied in thin layers until the treatment is complete. For consolidation, all that is required is funori added to the edges of flaking paint and the flakes held down to secure. We recently examined several objects treated between 1975 and 1980 and compared their present state to their original treatment records.
No color shifts were noted in the treatment records. Visual examination of the objects at the present time and comparison with previous photographs showed no color change. Funori continues to be used as a consolidant, adhesive, and poultice material, especially on Asian and Near Eastern works of art on paper. 5 CONCLUSIONSThe persistence over the centuries of funori as a useful adhesive, size, and consolidant is due to its availability, ease of preparation and use, and lack of toxicity. The scientific literature reveals much about funori's relation to other red seaweeds and gives insight as to why it has endured as a reliable material. Our research in the laboratory to find the best methods of preparation revealed that funori is flexible in preparation and applicable to most consolidation treatments that require a low-strength material. In addition to the funorans, our seaweed research examined the agars and carrageenans. Agar, solid at room temperature, is inappropriate for use as a consolidant. Our East Asian painting laboratory has been experimenting with carrageenan with good results, although, unlike funori, with its long history of use in conservation, carrageenan's durability over time is not yet known. Our reasons for investigating funori were to increase its purity, extract as much soluble material as possible, find the most effective and efficient method of preparation, and make a portable product with a long shelf life. ACKNOWLEDGEMENTSWe would like to thank all the companies and people who shared their experiences with funori, in particular Andrew Hare, supervisor of East Asian painting conservation, and John Winter, senior scientist at the Freer Gallery of Art and Arthur M. Sackler Gallery. We are grateful to the Andrew W. Mellon Foundation for its generous support of this research through its funding of the East Asian Paintings Research Program at the Freer Gallery of Art and Arthur M. Sackler Gallery. SUPPLIERS OF FUNORIAiko's Art Materials Import, Inc. 3347 N. Clark St. Chicago, Ill. 60657 phone: (773) 404-5600 fax: (773) 404-5919 e-mail: aikosart@aol.comwww.aikosart.com University Products 517 Main St. P.O. Box 101 Holyoke, Mass. 01040-0101 phone: (800) 628-1912 fax: (800) 532-9281 e-mail: info@universityproducts.comwww.universityproducts.com Talas 568 Broadway New York, N.Y. 10012 phone: (212) 219-0770 fax: (212) 219-0735 e-mail: info@talasonline.comwww.talasonline.com REFERENCESAiko's Art Materials Import. 1984. Directions for funori preparation. Chicago. Araki, C.1965. Some recent studies on the polysac-charides of agarophytes. Seaweed Symposium. Halifax: Pergamon Press. 3–17. Araki, C., and K.Arai. 1955. Studies on the chemical constitution of agar-agar. Pt. 18, Isolation of a new crystalline disaccharide by enzymatic hydrolysis of agar-agar. Bulletin of the Chemical Society of Japan40:329–45. Chapman, D. J.1980. Seaweeds and their uses. London and New York: Chapman and Hall. Coombs, E.2003. Personal communication. Washington, D. C. Duckworth, M., K. C.Hong, and W.Yaphe. 1971. The agar polysaccharides of Gracilaria species. Carbohydrate Research18:1–9. Evans, D.1984. Funori: A short description, recipe, and source. Book and Paper Group Annual3: 59. Freer Gallery of Art. 1984. Notes by Oriental Paintings Studio and Technical Laboratory. Freer Gallery of Art, Smithsonian Institution, Washington, D. C. Freer and Sackler Galleries. 1989. Martha Smith. Freer and Sackler Galleries. 2003. Martha Smith. Grabar, P.1959. Immunoelectrophoertic analysis. Methods of Biochemical Analysis7(36–37): 1–38. Grantham, S.2003. Personal communication. Washington, D. C. Higuchi, S.1979. Treatment on painting of sliding screen and wall panels to prevent exfoliation in Japan. Conservation of Far Eastern art objects. International Symposium on the Conservation and Restoration of Cultural Property. Tokyo: IIC. 69–77. Hirase, S., and Watanabe, K.1971. Fractionation and structural investigation of funoran. Seventh International Seaweed Conference, Sapporo, Japan. Tokyo: John Wiley and Sons. 451–54. Horie, C. V.1990. Materials for conservation: Organic consolidants, adhesives, and coatings. London: Butterworth.
Kariya, H.1995. The use of funori as a consolidant on matte paint layers: The conservation of a monumental polychrome sandstone Bodhisattva. Freer Gallery of Art, Smithsonian Institution, Washington, D. C. Keyes, K. M.1986. A method of conserving a work of art on a deteriorated thin surface laminate. Paper Conservator: Journal of the Institute of Paper Conservation10:10–17. Lobban, C. S., and M. J.Wynne. 1981. The biology of seaweeds. Los Angeles: University of California Press. Masako, K.1979. Japanese scroll paintings: A handbook of mounting techniques. Washington, D. C.: Foundation of the American Institute for Conservation. Masuda, K.1984. Vegetable adhesives used in the workshop of the hyogushi, restorer and mounter of Japanese paintings. Adhesives and consolidants: Preprints of the contributions to the Paris Congress. Paris: IIC. 127–28. Meer, W.1980. Gum agar. In Handbook of water-soluble gums and resins, ed. R.Davidson. New York: McGraw-Hill. 80–92. Michel, F., T.Geiger, and G.Teoh-Sapkota. 2002. Funori, ein japanisches Festigungmittel fur matte Malerei. Zeishrift fur Kunsttechnologie16:257–75. Oka, I.1988. A dry method for removing the backing papers from Japanese paintings. The conservation of Far Eastern art: Preprints of the contributions to the Kyoto Conference. Kyoto: IIC. 69–72. Percival, E.1970. Algal polysaccharides. In The carbohydrates: Chemistry and biochemistry, ed. W.Pigman, D.Hortan, and A.Herp. New York: Academic Press. 537–68. Percival, E., and R. H.McDowell. 1967. Chemistry and enzymology of marine algal polysaccharides. London and New York: Academica Press. Ren, D., H.Noda, H.Amano, and K.Nisikawa. 1994. Antihypertensive and antihyperlipidemic effects of funoran. Fisheries Science60:423–27. Ren, D., J. Z.Wang, H.Noda, H.Amano, and S.Ogawa. 1995. The effects of an algal polysaccharide from the Gloiopeltis-tenax on transplantable tumors and immune activities in mice. Planta Medica61:120–25. Saeki, Y., T.Kato, Y.Naito, I.Takazoe, and K.Okuda. 1996. Inhibitory effects of funoran on the adherence and colonization of mutans steptococci. Caries Research30:119–25. Seetharam, S.1999. Personal communication. Freer Gallery of Art, Smithsonian Institution, Washington, D. C. Sewer, P.1983. Agarose gels: Properties and use for electrophoresis. Electrophoresis4:375–82. Smith, C.1999. Personal communication. Holyoke, Mass. Smith, M.1990. The conservation of Islamic book pages. Book and Paper Group Annual9:119. Stancioff, D. J.1980. Reflections on the interrelationships between red seaweed source chemistry and uses. Tenth International Seaweed Symposium. Berlin: Walter de Gruyter. 113–21. Takano, R., K.Hayashi, S.Hara, and S.Hirase. 1995. Funoran from the red seaweed, Gloiopeltis complanata: Polysaccharides with sulphated agarose structure and their precursor structure. Carbohydrate Polymers27:305–11. Takano, R., H.Iwane-Sakata, K.Hayashi, S.Hara, and S.Hirase. 1998. Concurrence of agaroid and carrageenan chains in funoran from the red seaweed Gloiopeltis furcata Post. et Ruprecht (Cryptonemiales, Rhodophyta). Carbohydrate Polymers35:81–87. Takano, R., T.Yokoi, K.Kamei, S.Hara, and S.Hirase. 1999. Coexistence of agaroid and carrageenan structures in a polysaccharide from the red seaweed Rhodomela larix (Turner) C. Ag.Botanica Marina42:183–88. Takano, R., S.Yoshikawa, T.Ueda, K.Hayashi, S.Hirase, and S.Hara. 1996. Sulfation of polysaccharides with sulfuric acid mediated by dicyclohexylcarbodi-imide. Journal of Carbohydrate Chemistry15:449–57. Tsujimoto, K.1979. On my five years' experience of repairs on wooden statues in the United States. Conservation of Far Eastern art objects. International Symposium on the Conservation and Restoration of Cultural Property. Tokyo: IIC. 125–32. University Products. 2003–4. Directions for funori preparation. Holyoke, Mass. AUTHOR INFORMATIONJOSEPH R. SWIDER earned a BS in chemistry and art history from the University of Rochester in 1991 and a PhD in nuclear chemistry from the University of Maryland at College Park in 1998. During a portion of his graduate studies, he was employed in the Science Department in the National Gallery of Art in Washington, D.C. In 1998 he received a National Research Council postdoctoral fellowship at the National Institute of Standards and Technology in Gaithersburg, Maryland. Following this fellowship, he was employed as a research scientist at the Freer Gallery of Art and Arthur M. Sackler Gallery through a grant from the Andrew W. Mellon Foundation in scientific research of East Asian painting materials. Currently he is a senior research scientist for McCrone Associates, Inc. Address: McCrone Associates, Inc., 850 Pasquinelli Dr., Westmont, Ill. 60559 MARTHA SMITH has a BA in communications from the University of Maryland University College. She is a conservator at the Freer Gallery of Art and Arthur M. Sackler Gallery, caring for Near Eastern, South Asian, and Western works of art on paper. She has been preparing and using funori for more than 20 years. Address: Department of Conservation and Scientific Research, Freer Gallery of Art and Arthur M. Sackler Gallery, Smithsonian Institution, Washington, D.C. 20560-0707
 Section Index Section Index |