PLUTARCH'S REPORT ON THE BLUE PATINA OF BRONZE STATUES AT DELPHI: A SCIENTIFIC EXPLANATIONWALTER A. FRANKE, & MAGDA MIRCEA
4 THE BLUE COPPER COMPOUNDS OF PATINAAccording to Pausanias (1979), nine different artisans from all over the Greek world contributed to the Spartan Monument. It seems therefore extremely improbable that all the 37 bronze statues had the same unusual bronze composition unknown in Greek sculptural art or had been prepared artificially with a rare agent to produce a unique patination. In addition, recent studies usually deny that artificially applied patina ever existed on large classical bronzes (Born 1993). These conclusions would suggest that the outdoor statues of the Spartan Monument were coated with a natural blue patina due to deterioration
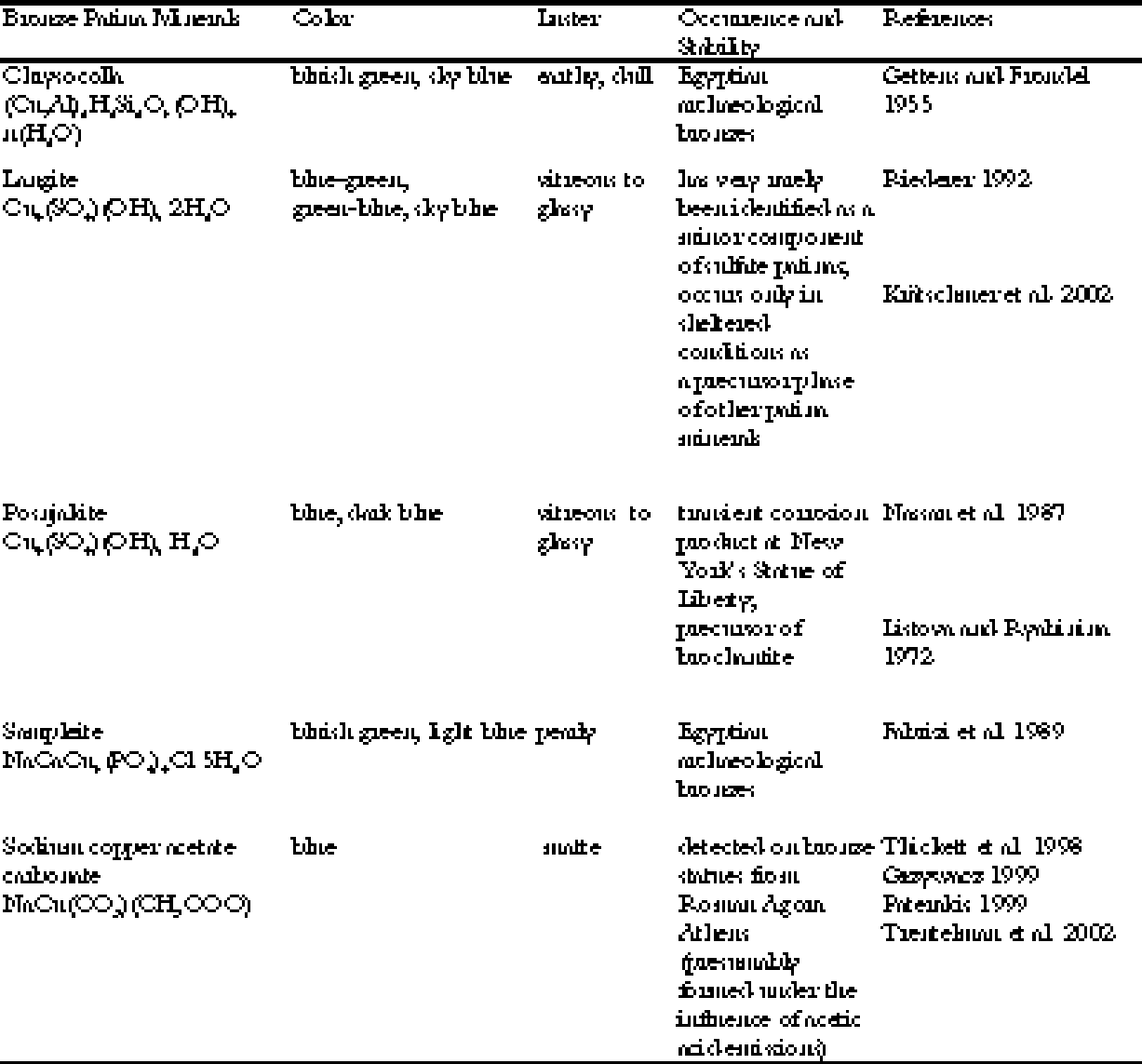
Bronze is an alloy of copper and tin, and in ancient times usually had only trace amounts of lead, but only copper compounds can be responsible for blue or green patina on the bronze. A full inventory of the copper corrosion products occurring in bronze patinas reveals that the majority of the some 30 known patina minerals are green, gray, or black, while only a few are blue or show at least a green with a bluish tint (see table 1). All the bronze patina minerals cited in table 1 are formed by the influence of soil solutions or by artificial treatment, or they are formed extremely rarely and in vestigial amounts as transient phases. Natural blue patinas are usually formed in the course of long burial in the earth, and not in an open-air environment. In the days of Plutarch, however, these statue offerings dedicated by Lysander had already been in place for 500 years. To make a further choice about the source of the patina, we can look to Plutarch's text to provide clues. The statues of the admirals were described as looking “like the sea” and “they stood there with the true complexion of the sea and its deepest depths” (Plutarch 1935, 260-61). Their patina was “shining with a deep blue tinge” (kyanou) (Plutarch 1935, 261). Kyanos is the Greek name for a dark blue enamel used to adorn armor in Homeric times. Kyanos also means “blue copper carbonate” or azurite (Liddell and Scott 1996). The use of the mineral azurite as a pigment after it had been crushed and ground into powder must have been known by Plutarch, since it was already recorded by Plinius the Elder in the first century A. D. (Gettens and FitzHugh 1993). The blue patina was also described as “agreeable and brilliant by blending light and luster with the blue” (Plutarch 1935, 269), “pleasant to the eye and
The luster of a material depends on its index of light refraction. All the blue minerals listed above will show a glassy, vitreous luster on a flat and even surface. There are a few exceptions: covellite shows a metallic luster, chrysocolla an earthy one, and sampleite a pearly one. These minerals can therefore be excluded. All blue patina minerals form patinas that consist of very tiny crystals, thus exhibiting a dull, lusterless surface. Only chalcanthite and azurite show a tendency to form bigger crystals that may show a vitreous to adamantine luster. Because chalcanthite is very easily dissolved and weathered in moist environments, excluding chalcanthite leads to the conclusion that the bright blue patina of the Spartan Monument at Delphi was probably due to an extensive coating with azurite. Since the ancient Greek name of the pigment azurite was kyanos, it might suggest a very correct but unconscious identification of the patina mineral by Plutarch himself, who used exactly this word to describe the color of the bronze statues. |